Interpretation
Eosinophilic (exudative) effusion with hemorrhage.
Explanation
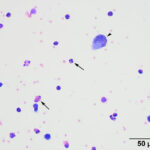
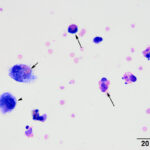
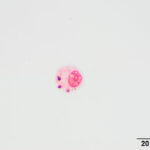
Direct and sediment smears prepared from the submitted fluid were highly cellular and composed of many leukocytes. The leukocytes consisted of approximately 52% eosinophils, 41% macrophages, 6% non-degenerate neutrophils, and 1% small lymphocytes. Based on the high nucleated cell count and percentages of leukocyte types, the fluid was interpreted as an exudative eosinophilic effusion. This type of effusion has not been reported in goats and the top differential diagnosis was a parasitic infection, based on the signalment and clinical signs (Question 1). Several macrophages were erythrophagocytic (Figure 2) and these cells frequently contained globules of golden yellow refractile to duller brown pigment in their cytoplasm, suspected to be a combination of hematoidin and hemosiderin, respectively (both products of hemoglobin breakdown, with hematoidin forming in hypoxic environments). Some of this material within the macrophages stained blue with a Prussian blue stain (Figure 3), indicating the presence of iron and confirming the presence of hemosiderin (iron-storage complex). Erythrophagia and hemosiderin/hematoidin within macrophages indicated prior in vivo hemorrhage (Question 2). Leukophagia of neutrophils was also noted as well as a rare multinucleated macrophage, suggesting underlying granulomatous inflammation or granuloma formation). No infectious organisms were observed.
Additional tests
Given the results of the pleural fluid analysis and the clinical suspicion of a parasitic pneumonia, a Baermann technique was performed on feces and was positive for Muellerius capillaris, which is a common lung worm found in wild and domestic goats and sheep. A fecal floatation analysis revealed a low burden of coccidia (336 oocysts/gram of Eimeria alijevi and Eimeria christenseni). The severe anemia corrected after the single transfusion (hematocrit of 33% on day 3 of hospitalization) and was suspected to be secondary to acute gastrointestinal blood loss (of unclear cause), which would also explain the mild thrombocytopenia, low protein and albumin concentrations, and the higher UN with lower creatinine concentrations after supportive fluid therapy. The inflammatory leukogram was attributed to a secondary bacterial pneumonia and not the parasitic infection. The respiratory alkalosis was due to hyperventilation from thoracic pain or hypoxemia and the high anion gap titration acidosis could be explained by hypoxia-induced anaerobic metabolism with L-lactate production. Hypoxia can be attributed to the severe anemia and potentially hypoxemia from respiratory disease (arterial blood gas analysis was not done). The cause for the liver injury and mild suspected cholestasis (increased direct bilirubin concentration with high GGT activity) was unclear, but it was likely from acute hypoxia with possible swelling of hepatocytes. Showering of the liver by bacterial endotoxins is also possible.
Follow up
The patient was continued on supportive care, dewormed with fenbendazole and moxidectin, and treated for secondary bacterial infections with penicillin G, florfenicol, and tulathromycin injections. Serial hemograms showed resolution of the inflammation by day 3 of hospitalization and a sustained hematocrit post-transfusion (37% on day 5 of hospitalization). The animal did develop a mild eosinophilia on days 3 and 5 of hospitalization (1.1. and 1.6 x 103/µL, respectively, RI: 0-0.8 x 103/µL), likely due to the verminous pneumonia. The lack of eosinophilia on bloodwork done the day after hospitalization could be due to the effects of concurrent stress (endogenous corticosteroids), which may also explain the mild monocytosis on point-of-care bloodwork at presentation. Serial biochemical panels showed resolution of all abnormal findings, with the exception of a persistently increased GGT activity and mild hyperbilirubinemia (total and direct), suggesting ongoing biliary hyperplasia and potential cholestasis. The animal developed a hyperglobulinemia on days 3 and 5 of hospitalization, compatible with an antigenic response to the infections. The patient was then released to the owner and referring veterinarian’s care. The last communication with the owner, 9 days later, indicated that the goat was doing well.
Discussion
Muellerius capillaris is one of a few lung worms known to infect small ruminants within North America. It has an indirect life cycle, involving a snail or slug as an intermediate host, and a small ruminant as the definitive host. Adult parasites commonly create nodules within the lung parenchyma of an infected animal and lay unsegmented eggs. The eggs hatch into first stage (L1) larvae, which gain access to the intestinal tract after being coughed up and swallowed. The L1 larvae are excreted within the feces and seek out an intermediate host. Once in the intermediate host, the L1 larvae matures to a L3 larvae. Small ruminants become infected after accidental ingestion of the infected intermediate host. The L3 larvae are released from the intermediate host into the gastrointestinal tract (GIT) of the ruminant. These larvae then penetrate the GIT wall, navigate to the mesenteric lymph nodes and migrate through the lymphatics to the heart and ultimately to the lung parenchyma. The L3 larvae mature into L4 larvae and then adult lung worms within lung nodules. The adults can live and continue to shed larvae for up to 4.5 years.1
Although Muellerius capillaris usually has a low pathogenicity and causes few clinical signs, compromised lung function, focal pneumonia, secondary bacterial infections, a reduction in weight, and an increase in mortality have been reported.1–3 M. capillaris infections are more prevalent and reportedly more severe in goats versus sheep;1,3,4 however, gross lesions and extent of the disease can vary between these two species.2 The most common gross lesion described is the formation of nodules, frequently on the caudal lung lobes, which appear to increase in number and size with the age of the animal.2,4 Infections can also create more diffuse and exudative lesions but this is a less common finding.1,2,4 These lesions can vary histologically from a few inflammatory cells surrounding parasite eggs, larvae, or adults to granuloma formation with necrotic centers of dying adult parasites.1–4 The clinical presentation of respiratory distress, pneumothorax, and pleural effusion with this patient is uncommon and was suspected to be a result of parenchymal rupture and subsequent effusion secondary to the parasitic pneumonia. Possible explanations could include increased worm burden and/or immune compromise of the patient due to comorbidities, such as intestinal parasitism or the severe anemia.
Treatment for M. capillaris has not been well established since most anthelmintics are not fully effective at eliminating the parasite.1,3 Common anthelmintics used in goats, including ivermectin and fenbendazole, can reduce fertility of the adult parasites, kill most of the adult worm population, and reduce larval fecal loads for a short period of time, but they cannot consistently eliminate all adult parasites or larval stages.1,3,5 Larvae that have evaded elimination are able to continue developing, which allows for repopulation of adult parasites, infection persistence, and continued larval shedding into the environment.1,3,5 Repeat administration of these anthelmintics is necessary to kill newly emerged adult parasites and larvae.1,5 A more recent report investigated the use of moxidectin in infected goats based on its efficacy for lungworm treatment in sheep.3 Moxidectin was shown to be safe and effective against M. capillaris infections due to the persistence of the drug within animals and the ability to kill new waves of developing larvae.3 A combination of fenbendazole and moxidectin was used in the treatment of this patient.
For more on respiratory disease in goats, Muellerius infection and images of the larvae in a tracheal wash, refer to the April 2018 Diagnostic Challenge.
References
- Panuska C. Lungworms of Ruminants. Vet Clin North Am Food Anim Pract. 2006;22(3):583-593
- Panayotova-Pencheva MS, Alexandrov MT. Some Pathological Features of Lungs from Domestic and Wild Ruminants with Single and Mixed Protostrongylid Infections. Vet Med Int. 2010;2010:e741062
- Vadlejch J, Makovický P, Čadková Z, Langrová I. Efficacy and persistent activity of moxidectin against natural Muellerius capillaris infection in goats and pathological consequences of muelleriosis. Vet Parasitol. 2016;218:98-101
- Valero G, Alley MR, Manktelow BW. A slaughterhouse survey of lung lesions in goats. N Z Vet J. 1992;40(2):45-51
- McCraw BM, Menzies PI. Treatment of Goats Infected with the Lungworm Muellerius capillaris. Can Vet J. 1986;27(8):287-290.
Authored by: Dr. Shelley Chu (resident), edited by Dr. Tracy Stokol