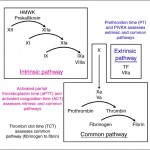
Screening coagulation assays are the “bread and butter” of secondary hemostasis testing and consist of the prothrombin time (PT), activated partial thromboplastin time (APTT) and the thrombin clot time. The thrombin clot time can be modified to measure fibrinogen concentration. The activated coagulation time (ACT) is an in-house point-of-care test that provides some information on secondary hemostasis and is useful as a screening tool for severe coagulation factor deficiencies. Additionally, a clotting assay that is sensitive to deficiencies of vitamin-K dependent factors (PIVKA assay) can also be performed, but has largely fallen out of favor. Also the dilute Russell’s viper venom time (dRVVT) can be used to evaluate clot formation with the common pathway but is not done routinely.
Laboratories offer different screening assays, but most hemostasis or coagulation profiles consist of the PT and APTT at the minimum, which are interpreted together based on the classic cascade model of secondary hemostasis. The combination of results from coagulation screening tests (PT, APTT and fibrinogen) can be used to determine the defect is in coagulation factors, as shown in the image and table below. It is important to note that not all laboratories are created equal with respect to coagulation testing. Some laboratories, such as the Comparative Coagulation Laboratory at Cornell University, optimize testing to be sensitive to coagulation factor deficiencies. This may involve dilution of a specific reagent, evaluating which method of clot detection is optimal for a given species, or using different clot activators or reagents to measure clotting times. Coagulation screening assays are only sensitive to factor deficiencies and not a hyperactive or excessively activated clotting system (hypercoagulability), i.e. only prolonged times are of diagnostic relevance. Short clotting times do not necessarily mean a hypercoagulable state and should not be interpreted as such. Indeed, short clotting times are often a laboratory or sampling error. Interpretation of all assays should only be done with respect to clinical signs. Normal clotting assays in a bleeding animal do not rule out a defect in secondary hemostasis (because the used assay may not be sensitive) and prolonged clotting times do not mean the animal is bleeding due to a defect in secondary hemostasis (particularly if there were sample collection issues). It is critically important to collect samples properly for hemostasis testing. There are also differences in how people interpret the results (with respect to reference intervals, percentage change compared to controls etc).
Methods of clot detection
Most screening coagulation assays are based on how rapidly fibrin clots form in patient samples. Since citrate anticoagulated plasma is used for most of these assays, calcium needs to be added, along with a clotting activator. After addition of these reagents, the time for fibrin clot formation is recorded. Different activators will trigger different pathways.
- Extrinsic pathway activator: This is tissue factor which is usually provided as an extract of brain (tissue thromboplastin) from rabbits or other species. Nowadays human recombinant tissue factor can be added instead.
- Intrinsic pathway activator: There are various substances that activate factor XII. Commonly used activators are kaolin, ellagic acid, glass (which is why blood clots in a red top tube) and siliceous earth (e.g. celite, diatomaceous earth).
- Common pathway activator: Viper venoms, e.g. Russell’s viper venom, can activate factor X. Thrombin can be used to measure the thrombin clot time (fibrinogen conversion to fibrin).
There are different methods to detect the formation of fibrin in these assays, including the visual tilt tube method, mechanical,photo-optical and viscoelastographic techniques. These not only differ in how they detect the clot but also on the provided results.
- Visual tilt-tube: The platelet-poor citrate anticoagulated plasma is placed in a tube, along with activators and the time to clot formation is recorded. This is the least sensitive of all methods (small fibrin strands may be missed as the eye can only pick up large clots), but is useful for ascertaining the nature of the clot, e.g. a poorly formed clot indicates problems with clot formation even if the time to clot formation is not abnormal.
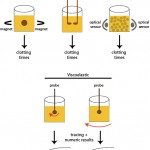
- Mechanical: There are different mechanical methods, but these detect fibrin formation because the fibrin restricts the movement of a metal ball (moved between two magnets) or probes inserted within the patient sample. The time to clot formation is provided. This is performed on platelet-poor citrate anticoagulated plasma.
- Photo-optical: This can be evaluated by the change in optical density (spectrophotometric) or turbidity (nephelometric) in the sample as a clot is formed. The results are recorded as the time to clot, formation however some analyzers also provide a curve of clot formation (called waveform analysis). Photo-optical assays can also be used to assess fibrinolysis (by addition of tissue plasminogen activator as well as a coagulation activator to the patient sample). Some of these assays may miss rapid clot detection in canine and feline plasma (which clot far quicker than human plasma) and may erroneously report out prolonged clotting times. This particularly occurs if the threshold for determining the time to start measuring clot formation cannot be changed on the instrument (i.e. shortened to pick up the quicker clotting times).
- Viscoelastic: With this technique, a probe is inserted into a cup containing the patient sample. Then either the probe is rotated within the cup (e.g. Sonoclot or ROTEM®; the technique is called thromboelastrometry when performed with the ROTEM®) or the cup is rotated around a fixed probe (when performed with TEG®, the technique is called thromboelastography, which gives similar measurements, albeit with different names, than thrombelastometry). Fibrin formation impedes movement of the probe in relation to the cup. This change in movement is detected electronically or optically and is converted into a curve, that reflects the actual kinetics and strength of clot formation, e.g. reaction time or time for start of fibrin formation and maximum amplitude or the strength of the clot. These assays can also examine clot breakdown or fibrinolysis. This technique, unlike mechanical and photo-optical methods, can be performed in whole blood or platelet-rich plasma, thus adding the critical cellular component to clot formation, which is lacking in the other methods. This is why they are often called “global” assays of hemostasis, because they not only assess secondary hemostasis, but are affected by platelet number and function, as well. More information on viscoelastographic clot detection is given separately.
Activated coagulation time (ACT)

The ACT is a useful screen for evaluation of the intrinsic and common pathways (see image above) and is performed in whole blood. The ACT relies on the patient’s platelets and calcium for provision of phospholipid to support the reaction. There are two ways of performing the test:
- Visual clot detection: Whole blood without anticoagulant is added to a special tube containing a clot activator and the tube is gently agitated at specific intervals until a clot is detectable. To minimize variability in test results due to temperature, the tube should be kept at 37°C in a heating block (although the armpit can serve as the heating block). Expected “normal” values depends on the tube, which can contain different activators, including kaolin (Hematologic technologies) or a combination of celine-kaolin-glass beads (MAX-ACT™, Helena Biosciences; the beads facilitate detection of the clot). Some people make up their own tubes, which is concerning regarding consistency of activator concentration in each tube. Previously, diatomaceous earth was used as a clot activator and published reference intervals for ACT in dogs and cats are based on this activator, however this is no longer available (and reference intervals are no longer relevant, unless still using this specific tube). Reference intervals for dogs and cats have been established for MAX-ACT™ tubes (See et al 2009). These intervals are specific for the latter tubes and cannot be extrapolated to other clot activating tubes (e.g. kaolin-based) and are the following:
- Dogs: 55-80 seconds.
- Cats: 55-85 seconds.
- Electrochemical sensing: There are also cartridges for hand-held point-of-care devices that contain clot activators for ACT measurement, such as kaolin and celite (iSTAT®, Abbott). Instead of fibrin formation, these actually measure thrombin generation, using thrombin-specific substrates (cleaved by thrombin). No studies have been performed in animals with this type of ACT.
Interpretation
- Deficiencies in intrinsic or common pathway factors: The ACT is an insensitive test (sensitive to single factor deficiencies of <10%) compared to the APTT (sensitive to deficiencies of < 30%, depending on the method), but it is sufficient to demonstrate severe clotting factor deficiencies, whether inherited (e.g. hemophilia A or factor VIII deficiency) or acquired (e.g. disseminated intravascular coagulation or anticoagulant rodenticides. Animals with the most common causes of acquired clotting factor deficiencies (namely anticoagulant rodenticide poisoning) will have prolonged ACT and it is a good screening tool for this purpose (but not specific for this toxicosis).
- Severe thrombocytopenia: Due to reliance on endogenous platelets for the reaction, platelet counts of < 10,000/µL may result in a mildly prolonged diatomaceous earth-based ACT (by 10-20 seconds). The effect of platelet count on other clot activators with the ACT is unknown.
Prothrombin time
The prothrombin time (PT, also called the one stage prothrombin time or OSPT) measures the activities of extrinsic and common pathway factors, with the exception of tissue factor (coagulation factor III), which is added as the clot activator along with calcium. Since tissue factor is never deficient in vivo, we can use it as an activator. Once these reagents are added, the time for clot formation is recorded. Canine and feline plasma clots rapidly in the PT (times are usually < 10 seconds), which can result in clot formation being missed, especially in photo-optical instruments optimized for use in human patients (who normally have PTs longer than 10 seconds). In addition, the short clotting times for the PT reduces the sensitivity of the assay. Some laboratories dilute their PT reagent to provide longer PT clotting times (in order to optimize sensitivity to factor deficiencies). A variety of sources of thromboplastin are available and these produce different times for the PT in the same species. Therefore, the PT should always be interpreted with respect to the species-specific reference interval provided by the laboratory and PT results from different laboratories are not directly comparable. Since a source of phospholipid is provided in the assay (as part of the tissue factor activator), this assay does not require platelets to support the reaction.
Test interpretation
Only a prolonged PT is a relevant finding (as long as it is not due to an artifact of detection method). The PT should never be interpreted in isolation but always in conjunction with the APTT (see diagnostic algorithm, table summary below and image above) and, ideally, fibrinogen concentration. This is because a low fibrinogen (<75 mg/dL in the dog and <50 mg/dL in other species) will prolong the PT (and APTT) independently of other clotting factor deficiencies (both tests rely on adequate fibrinogen to form the fibrin clot). Of all the tests performed at Cornell University, the sensitivity of the clotting assays to low fibrinogen are: TCT > APTT > PT.
- Reference intervals (from the Comparative Coagulation Laboratory at Cornell University)
- Dogs: 11-16 seconds
- Cats: 15-20 seconds
- Horses: 16-20 seconds
- Prolonged PT, normal APTT: Factor VII deficiency. The normal APTT supports that intrinsic and common factor pathway factors are not deficient. In general, factors need to be <30% of normal to result in prolonged clotting times.
- Artifact of collection: The PT may be prolonged with difficult venipuncture or if insufficient blood is collected into citrate. Over-dilution of plasma with citrate will prolong the clotting times. This may also occur in animals with a marked erythrocytosis (high hematocrit), resulting in recommendations to use a correction formula (for the degree of anemia (and adjust the citrate concentration) when collecting blood samples for coagulation testing in anemic animals, but this is not routinely done.
- Iatrogenic: Warfarin therapy. Warfarin inhibits vitamin K dependent factors (II, VII, IX, X).
- Inherited FVII deficiency: Reported in some breeds of dogs. They may not exhibit signs of hemorrhage. To confirm a specific factor VII deficiency, a modified PT using factor VII-deficient plasma is required (see specific factor assay below).
- Early vitamin K deficiency/antagonism: Since factor VII has the shortest half life of all factors (approximately 6 hours in the dog), the PT may be prolonged before the APTT, i.e. within 24 hours of toxicosis. However, animals will not usually show signs of hemorrhage until prothrombin (factor II) is deficient (by which time the APTT should be prolonged). The PT is also used for monitoring response to vitamin K1 therapy. Vitamin K deficiency can also occur with other disorders, including cholestastic liver disease and fat malabsorption.
- Disseminated intravascular coagulation: Some animals with DIC will have a long PT and normal APTT. This seems to be the case in cats versus dogs or horses. The PT tends to be normal in most dogs (up to 65%) with DIC.
- Synthetic liver failure: Since coagulation factors, including factor VII, are produced in the liver, the PT could be prolonged in liver failure, however usually the APTT is also prolonged.
- Inhibitors of factor VII: Antibody inhibitors of factor VII have not been reported in animals.
- Prolonged PT, prolonged APTT: Multiple factor deficiencies, affecting both factor VII (see list above) and intrinsic pathway factors (XII, XI, IX, X) or common pathway factor deficiencies. This can be due to multiple causes, including inherited factor X deficiency or acquired defects such as vitamin K deficiency or antagonism, DIC, liver failure and hypofibrinogenemia.
Activated partial thromboplastin time
The activated partial thromboplastin time (APTT) assay is an indicator of the function of coagulation factors in the intrinsic and common pathways. A surface activator (such as kaolin or ellagic acid, which activates factor XII), phospholipid, and calcium are added to citrated plasma at 37°C and the time to clot formation is recorded. Dogs have much shorter aPTT clotting times (15 – 20 seconds) than human patients (25 to 45 seconds), whereas healthy large animals have quite long aPTTs (> 40 seconds). The APTT is more sensitive to clotting factor deficiencies in the intrinsic and common pathways than the ACT (<30% versus <10% for the diatomaceous-based ACT) and also does not require platelets to support the reaction (due to added phospholipid in the reagent). Individual factor activities usually need to be less than 30% to prolong the aPTT (although this % is dependent on the reagents and methods used), however mild deficiencies in multiple factors in the intrinsic or common pathways can prolong the APTT, despite individual factors not being <30%.
Test interpretation
Only a prolonged APTT is a relevant finding (as long as it is not due to an artifact of detection method). The APTT should never be interpreted in isolation but always in conjunction with the PT (see diagnostic algorithm, table summary below and image above) and, ideally, fibrinogen concentration. This is because a low fibrinogen (<75 mg/dL in the dog and <50 mg/dL in other species) will prolong the APTT (and PT) independently of other clotting factor deficiencies (both tests rely on adequate fibrinogen to form the fibrin clot). The APTT is more sensitive than the PT to low fibrinogen (but less sensitive than the TCT).
- Reference intervals (from the Comparative Coagulation Laboratory at Cornell University)
- Dogs: 10-17 seconds
- Cats: 15-19 seconds
- Horses: 45-66 seconds
- Prolonged APTT, normal PT: The normal PT supports that factor VII is not deficient (tissue factor is never deficient), indicating the defect is in the intrinsic pathway (factors XII, XI, IX, VIII) or contact (prekallikrein, high molecular weight kininogen) factors. In general, factors need to be <30% of normal to result in prolonged clotting times.
- Artifact of collection: The APTT is more sensitive to in vitro activation of clotting factors than the PT and may be prolonged (or falsely shortened or falsely normalized) with difficult venipuncture. The APTT may also be prolonged if insufficient blood is collected into citrate. Over-dilution of plasma with citrate will prolong the clotting times. This may also occur in animals with a marked erythrocytosis (high hematocrit), resulting in recommendations to use a correction formula (for the degree of anemia (and adjust the citrate concentration) when collecting samples for hemostasis testing in anemic animals, but this is not routinely done.
- Iatrogenic: Heparin therapy – heparin potentiates the antithrombotic action of antithrombin (inhibits multiple factors, but particularly factors II and X). Treatment with unfractionated but not fractionated (low molecular weight) heparin will prolong the APTT without affecting the PT. The APTT can be used to monitor treatment with unfractionated heparin – the target is an APTT that is prolonged 1.5-2 x normal, but animals may exhibit signs of bleeding at doses reaching this target level.
- Inherited deficiency of intrinsic or contact factors: Of these disorders, hemophilia A (factor VIII deficiency) is the most common disorder, followed by inherited deficiency of factor IX (hemophilia B) in dogs. Factor XII deficiency is common in cats and the likely cause of a prolonged APTT in an asymptomatic cat (factor XII is not required for physiologic hemostasis). Other deficiencies are far less common. Deficiencies in contact factors (e.g. prekallikrein) are rare and may not result in excessive hemorrhage (contact factors are not required for physiologic hemostasis). To confirm a specific factor deficiency, additional specific factor assays are required (see below).
- Disseminated intravascular coagulation: Many animals with DIC, particularly dogs and horses, will have a long APTT and normal PT (the APTT is more sensitive to DIC in these species).
- Synthetic liver failure: Since coagulation factors (except factor VIII and vWf) are produced in the liver, the APTT could be prolonged in liver failure.
- Inhibitors: Anti-phospholipid antibodies (e.g. in dogs with systemic lupus erythematosis) or antibodies that are acquired secondary to transfusion therapy in dogs with severe inherited factor deficiencies (e.g. factor VIII antibodies can develop in dogs treated for hemophilia A, making it difficult to keep treating the animals) or other diseases can prolong the APTT. These inhibitors can be detected by diluting the sample with normal plasma. The prolonged clotting times may shorten (from concurrent dilution of inhibitors) but should still be prolonged whereas a factor deficiency alone will be corrected by addition of normal plasma.
- Other interferents: In one study, patients with high C-reactive protein concentrations had a prolonged APTT, particularly with certain APTT reagents (e.g. STA Cephascreen). The prolongation was reduced with added phospholipid, suggesting that C-reactive protein interferes with the phospholipid provided in the assay (van Rossum et al 2012).
- Prolonged PT, prolonged APTT: Multiple factor deficiencies, affecting both factor VII and intrinsic pathway factors (XII, XI, IX, X) or common pathway factor deficiencies (Factors X, V, II and fibrinogen). This can be due to multiple causes, including inherited factor X deficiency or acquired defects such as vitamin K deficiency or antagonism, DIC, liver failure and warfarin therapy.
- Vitamin K deficiency/antagonism: Once factor IX, II (prothrombin) and X are deficient, the APTT will be prolonged. This can take 48 hours in dogs after anticoagulant rodenticide toxicosis. By the time prothrombin is deficient, the animal will exhibit signs of hemorrhage. Vitamin K deficiency can also occur with cholestatic liver disease among other disorders.
Thrombin clot time
The thrombin clot time (TCT) is a direct measurement of functional fibrinogen, because it assesses the ability of added thrombin to form a fibrin clot. The TCT is the time taken for a standardized thrombin solution to convert fibrinogen to fibrin to form a clot. Clot formation is decreased (which will prolong the TCT) if there is a deficiency of (hypofibrinogenemia) or abnormal (dysfibrinogenemia) fibrinogen. Furthermore, any other factors inhibiting fibrin polymerization will prolong this test, without there being any abnormalities in fibrinogen. This is the technique used by the Comparative Coagulation Laboratory at Cornell University for measurement of fibrinogen in citrated plasma samples (and is usually a part of their routine coagulation panel).
Test interpretation
Only a prolonged TCT is a relevant finding (as long as it is not due to an artifact of detection method). The TCT should never be interpreted in isolation but always in conjunction with the PT (table summary below and image above) and APTT. However, the TCT is more sensitive than the APTT or PT to low fibrinogen. The TCT may be prolonged under the following situations:
- Reference intervals (from the Comparative Coagulation Laboratory at Cornell University)
- Dogs: 5-9 seconds
- Cats: 5-8 seconds
- Horses: 5-9 seconds
- Prolonged values
- Artifact: Contamination with heparin anticoagulant or heparin therapy.
- Iatrogenic: Heparin therapy inhibits thrombin.
- Inherited hypofibrinogenemia or dysfibrinogenemia: This is uncommon.
- Acquired hypofibrinogenemia: This can be due to DIC where there is consumption of fibrinogen (hypofibrinogenemia) or synthetic liver failure which can result in decreased fibrinogen production. Since fibrinogen is an acute phase protein, concentrations reflect the effects of production (stimulated by the primary disease) and consumption and may be high (production outstrips consumption), normal (production is balanced by consumption) or low (consumption exceeds production) in DIC. The most common findings are normal or increased fibrinogen with low fibrinogen being seen in <30% of cases (in dogs at any rate).
- Acquired dysfibrinogenemia: The TCT may be prolonged if there are inhibitors of fibrin polymerization, including paraproteins and high concentrations of fibrin(ogen) degradation products (FDPs), the latter of which occurs in DIC. High concentrations of abnormal immunoglobulins can be seen with neoplasms involving plasma cells (multiple myeloma) or B cells (lymphoma, chronic lymphocytic leukemia).
- Inhibitors of thrombin: This is usually due to heparin (unfractionated) but acquired antibodies could also inhibit thrombin.
Specific factor assays
There are currently two methods to determine specific factor activities. The first is based on the ability of patient plasma to correct the prolonged clotting times of a plasma with a known factor deficiency and the second is based on the ability of activated factors to cleave a specific chromogenic substrates.
- Clotting assays: Deficiencies of specific factors are suspected in patients with prolonged clotting times that shorten or correct when diluted in plasma from a normal patient or plasma pool. This feature is used to quantify specific factor concentrations using a modified PT or APTT as follows. The ability of the patient’s plasma to normalize the prolonged clotting time of specific factor-deficient plasma is determined. For example, when patient plasma is added to factor VIII deficiency plasma and the prolonged APTT does not correct (it may shorten somewhat), the results indicate a factor VIII deficiency or hemophilia A. The degree of deficiency can then be quantified by comparing the clotting time of the patient plasma in factor-deificent plasma to a standard curve, produced from the serial dilution of a species-specific standard plasma pool, which is designated as having a specific factor activity of 100% when undiluted (and would have a normal PT or APTT). Therefore, the activity of the coagulation factor being measured is reported as a % of this standard pool in relation to the PT or APTT achieved when the patient sample is added to the factor-deficient plasma. In general, healthy animals have specific factor activities > 60%. Animals with specific factors < 30% are likely to demonstrate clinical signs of hemorrhage. In the example above, a factor VIII activity of < 30% is consistent with a diagnosis of Hemophilia A, with <1% being severe, 1-5% being moderate, and 5-30% being a mild deficiency (bleeding symptoms do correlate to severity of the deficiency).
- Chromogenic assays: These assess the ability of the specific factor to cleave a chromogenic-linked substrate. For example, in the factor VIII chromogenic assay, factor VIII in the patient sample is activated by a reagent containing thrombin and activated factor IX. The activated factor VIII then accelerates the factor IXa-mediated conversion of factor X to factor Xa. The activity of activated factor X is assessed by hydrolysis of a chromogenic substrate that is specific for factor Xa (and will not be cleaved by FIXa or FVIIIa). The intensity of color produced is proportional to the activity of factor Xa and thus to factor VIII activity. There are several chromogenic assays for coagulation factors, inhibitors and components of the fibrinolytic system. Few of these have been validated in the dogs, whereas several have been validated in horses. These chromogenic assays eliminate the need for specific factor-deficient plasma, particularly species-specific deficient plasma, which is difficult or impossible to obtain.
PIVKA
The vitamin K-dependent coagulation factors (FII, FVII, FIX, FX) are produced in the liver as nonfunctional precursors. These precursors are activated, in the presence of vitamin K, by carboxylation of their glutamic acid residues. This is achieved with the aid of an enzyme called γ glutamyl carboxylase. Carboxylation of these amino acid residues increases the negative charge of the protein and allows these coagulation factors to bind calcium, which is essential for the adhesion of these factors to platelet phosphatidylserine and formation of highly active enzymatic complezes (which is necessary for fibrin formation). In the absence of vitamin K, there is an increase in these non-functional factors in the circulation, as well as a depletion of the functional activated coagulation factors. The inactive precursors that accumulate in the circulation are called Proteins Induced by Vitamin K Antagonism or Absence (PIVKA).
The PIVKA test or thrombotest is actually a modification of the PT and does not measure the inactive precursors per se. The test uses diluted plasma (which creates longer clotting times than the PT) and a specific thrombotest reagent, which is reported to be most sensitive to deficiencies in factor X. Although claims have been made in the veterinary literature that this test is sensitive to both an increase in PIVKAs (which are supposed to inhibit the reaction) as well as a decrease in the functional coagulation FII, VII, and X (especially FVII and X), there is no proof that PIVKAs actually inhibit the reaction. Therefore, the PIVKA test should be considered a modified PT that detects deficiencies in FII, VII, and X and the term thrombotest versus PIVKA should be used when referring to this specific clotting assay. Because the thrombotest clotting times are longer than the PT, the test may be more sensitive than the PT for abnormalities in these factors, however this was not supported by a study measuring thrombotest, PT and APTT in dogs with various hemostatic disorders. Furthermore, the test is only offered by certain laboratories and the turnaround time is longer than the PT, therefore measurement of the thrombotest offers no advantage over the PT. The test has gone out of favor in veterinary medicine and is rarely used anymore. In human patients, the nonfunctional noncarboxylated coagulation proteins are measured specifically using immunologic tests.
Test interpretation
Results are interpreted similarly to the PT. Decreased thrombotest times are not diagnostically relevant, however difficult venipuncture may activate clotting factors leading to a falsely normal (instead of prolonged) thrombotest. Results are normal in dogs with inherited deficiencies of coagulation factors VIII, IX, and XI, and prekallikrein. Prolonged values could be due to the following:
- Artifact of collection: The thrombotest can potentially be affected by the same variables as the PT and APTT (difficult venipuncture, over-dilution).
- Iatrogenic: Warfarin therapy. Warfarin inhibits vitamin K dependent factors (II, VII, IX, X).
- Inherited FVII deficiency
- Vitamin K deficiency/antagonism: As indicated above, the thrombotest may be prolonged earlier than the PT.
- Disseminated intravascular coagulation: Some animals with DIC will have a prolonged thrombotest, depending on the severity of factor deficiencies. PT and normal APTT. This seems to be the case in cats versus dogs or horses. The PT tends to be normal in most dogs (up to 65%) with DIC.
- Synthetic liver failure
Dilute RVVT
The dilute Russell Viper Venom Test/Time (dRVVT) is used as a screening tool for the presence of anti-phospholipid antibodies or lupus anticoagulants. The test uses Russell’s viper venom (Daboia species) to activate factor X, which in the presence of factor V and phospholipid, converts prothrombin to thrombin. Clot formation is detected using any of the above methods. With the dilute test, the venom and phospholipid are diluted so that phospholipid concentrations are rate-limiting. The presence of antibodies to phospholipid decrease the availability of phospholipid to support the reaction, thus lengthening the clotting time. However coagulation factor deficiencies in the common pathway would also prolong times, so to differentiate between antiphospholipid antibodies and coagulation factor deficiency, normal plasma or excess phospholipid could be added. If the dRVVT corrects with normal plasma, then a coagulation factor deficiency is likely and assays for specific factors are indicated. If normal plasma does not correct the prolonged time but excess phospholipid (which saturates the antibody) does, then this would support the presence of an antiphospholipid antibody.
Interpretation summary
The table below provides a brief summary of using combination of screening coagulation test results – the PT, APTT, and TCT – to help determine where the defect is in secondary hemostasis. The list of disorders is not exhaustive.
| PT | APTT | TCT | Location of defect | Associated disorders | Notes |
| Normal | Normal | Normal | Likely not a disorder of secondary hemostasis | vWD, thrombopathia, Scott syndrome, fibrinolytic defects, hypercoagulable syndromes (e.g. non-overt DIC) | Some secondary hemostatic defects, e.g. mild hemophilia A, may be missed if non-optimized screening tests are used. Normal screening coagulation results do not rule out a hemostatic disorder |
| ↑ | Normal | Normal | Extrinsic pathway (factor VII) | Inherited factor VII deficiency, early vitamin K deficiency or antagonism, DIC, warfarin treatment, liver disease (cholestatic, liver failure) | A prolonged PT in isolation supports factor VII deficiency (tissue factor is part of the reagent in the PT assay) |
| Normal | ↑ | Normal | Intrinsic pathway (deficient factors XII, XI, IX, VIII) | Inherited deficiencies, DIC, liver disease, unfractionated heparin therapy, artifact of collection | The APTT is more sensitive to collection artifact than the PT. Times can be prolonged or shortened with poor venipuncture technique |
| Normal | Normal | ↑ | Hypofibrinogenemia or dysfibrinogemia | Isolated fibrinogen production defect, inhibitors of fibrin polymerization (high FDP, paraproteins), inherited fibrinogen disorder | This is an unusual result, although the TCT is more sensitive to isolated hypofibrinogenemia than the APTT or PT (with the assays used at Cornell University). Usually, causes of hypofibrinogenemia (DIC, liver failure) will result in additional factor deficiencies and prolong the PT and/or APTT |
| ↑ | ↑ | N | Common pathway (factors X, V, II) or defects in intrinsic and extrinsic pathway | Overt DIC (FDPs not high enough and fibrinogen not low enough to prolong the TCT), vitamin K deficiency or antagonism, liver disease (cholestatic, failure), inherited defect in common pathway factors (excluding fibrinogen), warfarin and unfractionated heparin therapy., over-dilution of blood (too little blood for the citrate, standard collection of blood in a patient with severe erythrocytosis), incorrect sample handling (see collection guidelines, factors are unstable) | Expect the platelet count and AT activity to be low in overt DIC and normal in vitamin K deficiency or antagonism (there are always exceptions) in dogs. Animals with overt DIC should have an initiating disease (e.g. inflammation, neoplasia, bacterial sepsis). D-dimer may be high with overt DIC and non-DIC causes of prolonged PT and APTT (increased intravascular and extravascular fibrinolysis). |
| ↑ | ↑ | ↑ | Common pathway (factors X, V, II, I) or defects in intrinsic and extrinsic pathway | Severe hypofibrinogenemia alone (<50 mg/dL dog, <75 mg/dL other species – see causes above), overt DIC, synthetic liver failure, unfractionated heparin therapy, possibly over-dilution of blood, inappropriate storage of sample | Fractionated heparin will not prolong clotting times (inhibits FXa primarily, not thrombin). |
Related links
- Comparative Coagulation Laboratory in the Animal Health Diagnostic Center at Cornell University: Information on fibrinogen testing.
