The red blood cell count on the routine CBC is the concentration of red blood cells, expressed in millions/µL of whole blood. While red blood cell counts can be performed by manual techniques, such as a hemocytometer, these are time-consuming and inaccurate. We do, however, use them for counting RBC in fluids with low cell counts, such as cerebrospinal fluid (CSF). Automated counts are most commonly performed using electronic impedance or laser light scattering (flow cytometry). The latter is the technique used by the hematology analyzer at Cornell University, except under specific situations (see below).
Method of measurement
Manual counts
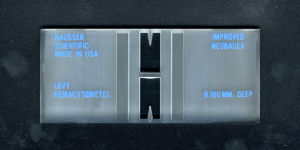
Manual cell counts (RBC, nucleated cells or platelets) are performed using a hemocytometer. This is specifically done on body cavity fluids that are poorly cellular (<1000 cells/uL) because most automated analyzers (impedance or laser-based) are insensitive to such low values. This is particularly the case with cerebrospinal fluid, where the red blood cell count should be low (unless there is hemorrhage into the fluid or concurrent blood contamination during collection). For CSF, the fluid is dispensed directly into the counting chamber and both sides (all squares) are counted (and averaged) to obtain a RBC count, in cells/uL (versus millions/uL for blood or thousands/uL for other body cavity fluids).
Impedance counters
This technique uses changes in electrical resistance to count cells and provide an assessment of cell volume (depending on the counter). It is used to measure RBC in blood samples as well as samples of body cavity fluids (peritoneal, pleural). With this technique, the sample is first diluted, then counting is performed by drawing the cells through an aperture of the instrument. Each cell causes a change in electrical resistance as it passes the aperture, and this pulse is detected and amplified by the instrument. The sensitivity (or thresholds) can be adjusted so that platelets (smaller than red blood cells) are not counted; WBC are counted as well as RBC, but white blood cell numbers (in thousands/µL) are too low to cause significant error in the red cell count (in millions/µL). The amplitude of the pulse is proportional to cell size and, in some analyzers, this is the method used for determining the mean corpuscular volume (MCV), which is then a calculated value versus one that is directly measured. We use an impedance Coulter Z2 counter as our back-up analyzer. This performs cell counts and yields a printout (if we desire) of the variation in volume of the counted cells (as a frequency distribution curve or histogram). We use this cell counter when our automated analyzer is out of commission or inaccurate. The latter scenario most frequently occurs in camelids with iron deficiency anemia. This is because the microcytic RBC fall below the threshold of our automated hematology analyzer, which cannot be adjusted to compensate for the smaller RBC. Thus, the RBC count is erroneously low, leading to false increases in the MCV (which is still calculated in this species). However, we can adjust (lower) the threshold on the impedance counter to include the smaller RBC, thus obtaining a more accurate RBC count in this setting. The impedance counter is also what we use to obtain nucleated and red bolo cell counts in body fluids, other than blood, e.g. joint fluids, peritoneal fluids, because these fluids cannot be analyzed through our automated analyzer (these samples may plug up the instrument).
Flow cytometry counters
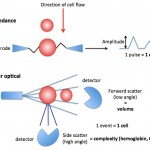
With the hematology analyzer used at the Clinical Pathology Laboratory at Cornell University, red blood cells are sphered in a diluent and then passed through a laser light detector. The cells scatter light (at different angles) which is detected by the instrument (see image to the right). The laser detects the number of cells (as events passing through the laser), cell volume (using low angle scatter) and internal content, i.e. hemoglobin concentration (using high angle scatter) by light scatter.
Units of measurement
RBC counts are expressed as millions/uL of the blood (SI units are x 109/L). The conversion formula to SI units is 1:1.
Sample considerations
Sample type
Whole blood, body cavity fluids
Anticoagulant
EDTA is the preferred anticoagulant. Although citrate can be used, the volume of citrate in the tube (10% of the collection volume) will dilute the RBC count accordingly (although a correction formula accounting for the 10% dilutional effect is not always accurate). Heparinized whole blood can also be used.
Stability
Red blood cell counts are optimally stable for 24 hours at 4°C. Red blood cells start to lyse with storage, resulting in false decreases in the count. This will occur quickly in lipemic samples.
Interferences
- Lipemia, icterus: No effect on RBC count.
- Hemolysis: Artifactual (in vitro) hemolysis falsely decreases the RBC count. In an animal with true intravascular hemolysis versus in vitro hemolysis, the RBC count will be low, but this is what is available to carry oxygen in the animal.
Test interpretation
Increased values
- Artifact: Loss of water from the tube (e.g. incomplete capping).
- Physiologic: Some breeds of dogs may have higher RBC counts, hematocrit and hemoglobin concentration, such as Dachshunds (Torres et al 2014), Greyhounds (Shief et al 2007, Campora et al 2011) and Whippets (Uhriková et al 2014).
- Pathophysiologic
- Relative change to blood water: Dehydration, splenic contraction secondary to epinephrine (horses).
- Absolute increase in RBC mass or erythrocytosis: Stimulated by erythropoietin (secondary erthrocytosis) or erythropoietin-independent (primary erythrocytosis, e.g. polycythemia vera)
Decreased values
- Artifact: Hemolysis of RBC due to sample collection or storage (in vitro hemolysis). In this setting, the measured hemoglobin is the most accurate measure of the animal’s oxygen carrying capacity.
- Pathophysiologic:
- Relative change to blood water: Over-dilution with fluids, splenic relaxation (anesthetic agents, tranquilizers).
- Absolute decrease in RBC mass: Indicates a true anemia, due to hemorrhage, hemolysis (intravascular, extravascular) or decreased production. Multiple mechanisms may be operative.
