Synonyms
Non-esterified fatty acids (NEFA), free fatty acids, unsaturated fatty acids
Physiology
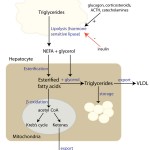
Non-esterified (“free” or unsaturated) fatty acids (NEFAs) are the major component of triglycerides (the fat stores in the body), which consist of three fatty acids linked to a glycerol backbone. In healthy animals (when they are eating or not in energy-deficient states), normal NEFAs mainly come from breakdown of triglycerides ingested in the diet through chylomicrons (lipoprotein lipase liberates NEFA off chylomicron remnants). However, under fasting conditions or states of negative energy balance, the main source of NEFA is hydrolysis of fat stores in the body and NEFAs are used primarily as a marker of negative energy balance.
Hydrolysis of stored triglycerides (fat) in adipose tissue by hormone-sensitive lipase liberates NEFAs and glycerol. Hormone-sensitive lipase (which is found within the cytosol of adipocytes) is stimulated by various hormones, including glucagon (which is released from α-cells in pancreatic islets in response to low glucose) and corticosteroids. Once liberated from fat or chylomicrons, NEFAs can be used as an energy source by many tissues, including skeletal muscle and hepatocytes. In hepatocytes, their fate differs depending on energy needs, hormone balance and substrate availability, i.e. they can be used for energy production (via Kreb’s cycle), re-packaged into triglycerides and exported as very low density lipoproteins (VLDL) or stored within the liver as triglycerides (which in excess can cause hepatic lipidosis) or converted to ketones.
Methodology
The primary method for measuring the concentration of NEFAs in serum is a colorimetric enzymatic method, which is the method used at Cornell University.
Reaction type
Blanked end-point reaction. Blanking decreases the effect of hemolysis on NEFA concentrations.
Procedure used at Cornell University
In the first step of this three stage reaction, acyl-CoA synthetase (ACS) catalyzes the acylation of NEFAs to (coenzyme A) CoA resulting in the formation of acyl-CoA. Hydrogen peroxide is then generated from the oxidation of acyl-CoA through the catalytic action of acyl-CoA oxidase (ACOD). Lastly, hydrogen peroxide in the presence of peroxidase (POD) allows for the oxidative condensation of 4-aminoantipyrine with 3-methyl-N-ethyl-N-(β-hydroxyethyl)-aniline (MEHA) to form a blue-purple end product with an absorbance maximum at 550 nm. NEFA concentration is directly proportional to the measured optical density of the dye product.
Reactions are shown below:
NEFA+ ATP + CoA acyl-CoA synthesase > Acyl-CoA + AMP + pyrophosphate
Acyl-CoA + O2 + acyl-CoA oxidase > 2,3-trans-Enoyl-CoA + H2O2
2H2O2 + N-ethyl-N (2hydroxy-3-sulphopropyl) m-toluidine + 4-aminoantipyrine peroxidase > purple complex+ 4H2O
Units of measurement
The concentration of NEFAs is measured in mEq/L (conventional units) or mmol/L (SI units). The unit conversion is obtained from the following formula:
mEq/L x 1= mmol/L
Sample considerations
Sample type
Serum or EDTA plasma. Serum/plasma should be separated from cells ASAP after collection and the serum/plasma placed in a separate tube. Similarly, corvac (serum-separator) tubes are not recommended, because values are slightly (but significantly) higher in these tubes compared to non-anticoagulant (red top) tubes.
Anticoagulant
EDTA. Values are less stable in heparin, which also yields higher baseline concentrations than EDTA or serum and NEFA values increase with storage (Stokol and Nydam 2005). The manufacturer of the assay also states that heparin interferes with the assay.
Stability
Stable in bovine serum or EDTA plasma at 4°C for 72 hours. Stable frozen in bovine serum for 1 month (-70°C). Values increased within 24-48 hours of storage at 24°C in serum or EDTA plasma. NEFA concentrations increase rapidly in heparin after storage. (Stokol and Nydam 2005).
Recommendations for sample collection in transition dairy cows
- Anticoagulant: Red top (serum) or purple top (EDTA). Avoid heparin (green top) and corvac (serum separator) tubes.
- Handling: Separate from cells ASAP, keep cool to minimize false changes in results (NEFAs are particularly unstable), submit ASAP to the laboratory.
- Time of collection: Sample cows as they are coming into the feeding stalls (see below).
- Prepartum NEFAs: Collect samples from cows 2-14 days before calving.
- Postpartum NEFAs: Collect samples from cows 3-14 days in milk. Measurement of both postpartum NEFAs and BHB is performed in a transition cow energy profile for assessment of energy status in lactating cows at the Clinical Pathology Laboratory in Cornell University.
- Number of cows: A minimum of 12 animals per herd should be sampled for herd level testing (test description in test interpretation section below). This can be a mixture of heifers and >2 parity cows.
- Pooled samples: Pooling of individual samples from cows to assess energy status of a herd is NOT recommended. Studies at Cornell University have shown that results from pooled samples are less sensitive than individual cow testing (minimum 12 animals per herd) when using prepartum or postpartum NEFA for the detection of excessive negative energy balance in transition dairy cows.
Interferences
- Lipemia, icterus: Unknown effect (rare in cattle).
- Hemolysis: Depending on the method used by the laboratory, hemolysis will either decrease or increase NEFA values. In the blanked method used at Cornell University, severe hemolysis (hemolytic index > 800 units) will mildly decrease NEFA concentrations in bovine blood. Lipemic and icteric interferents do not affect the NEFAs concentration substantially with the methods used by Cornell University.
- Excitement/exercise/stress: These conditions, which induce catecholamine or ACTH release, increase NEFA concentrations by stimulating lipolysis through hormone sensitive lipase. These conditions should be minimized when collecting blood samples for NEFA measurement.
- Timing of sample collection: NEFA values are likely to be higher in cows that are coming in for daily feeding (likely to be in some degree of negative energy balance) versus those cows which have just been fed (lipolysis will be inhibited in these latter cows). Collection just before feeding is recommended.
Test interpretation
NEFA testing is mostly done in dairy cattle to determine if the herd is in excessive negative energy balance. It is used more as a herd level than individual animal level test and results are interpreted with respect to a cut-off concentration or percentage of animals with values above the cut-off versus values above the upper reference limit. NEFA measurement can be done in any other species as a marker of excessive negative energy balance. This is most frequently done in camelids (which develop hepatic lipidosis under these circumstances), other ruminants (e.g. goats, sheep) to determine if they are at risk of ketosis, and horses (with equine metabolic syndrome). Note that concentrations below the reference limit are not clinically relevant.
Increased concentrations
- Physiologic: Exercise induces release of catecholamines and ACTH, which stimulates the hydrolytic activity of hormone-sensitive lipase to promote fat mobilization in order to meet excess energy requires. This process increases the concentration of NEFAs in blood.
- Breed differences: Several studies have shown that NEFAs are lower in Jersey cows post-partum versus Holstein cows, hence guidelines developed for Holstein cows (see below) may not be applicable to Jerseys (Rastani et al 2001, French 2006, Guretsky et al 2006, Brown et al 2012).
- Negative energy balance in dairy cows: Dairy cows in the periparturient (transition) period are always in a state of negative energy balance due to high energy demands from the developing fetus and milk production (particularly with the emphasis on selection for high milk-producers). However, this state of negative energy balance can be excessive and affected cows are at risk of gastrointestinal (displaced abomasum), metabolic (clinical ketosis), and infectious (e.g. metritis) diseases in the early postpartum period. Thus, dairy practitioners frequently monitor dairy herds for excess negative energy balance by testing for NEFAs, either alone in pre- or postpartum cows or as a component of a metabolic profile in postpartum cows. Results of these tests can be interpreted at the individual cow level (i.e. a NEFA value above a certain cut-off indicates excess negative energy balance) or at the herd level (i.e. a proportion of tested cows have NEFA values over a certain cut-off value). Identification of excess negative energy balance in individual cows (and more importantly) in the herd indicates the need for changes in nutrition (e.g. increase bunk feed space, increase energy density of ration) and transition cow management to decrease energy demands and stresses on transition cows. The following interpretation guidelines are based on studies done at Cornell University and are valid for samples collected from ‘at risk’ TMR-fed Holstein cows between 2-14 days precalving (prepartum NEFAs) or 3-14 days post-calving (postpartum NEFAs). We recommend sampling at least 12 ‘at risk’ cows when evaluating total mixed ration (TMR)-fed herds for subclinical ketosis (Ospina et al 2013 review).
- Cow level testing
- Prepartum NEFAs: There is an increased incidence of postcalving diseases (displaced abomasum, metritis/retained placenta and clinical ketosis), decreased milk yield and decreased reproductive performance in the first 30 days in milk in Holstein dairy cows (fed TMR) with NEFA values > 0.30 mEq/L when tested 2-14 days before calving.
- Postpartum NEFAs: There is an increased incidence of postcalving diseases (displaced abomasum, metritis/retained placenta and clinical ketosis), decreased milk yield and decreased reproductive performance in the first 30 days in milk in Holstein dairy cows (fed TMR) with NEFA values > 0.60-0.70 mEq/L when tested 3-14 days after calving (Ospina et al 2010, Ospina et al 2010). In the Cornell studies, postcalving NEFAs were actually a better predictor of than postcalving β-hydroxybutyrate concentrations or precalving NEFAs for postcalving disease (Ospina et al 2010).
- Herd level testing
- Prepartum NEFAs: At the herd-level, there is a significantly increased risk of post-calving metabolic and infectious diseases, decreased milk production or decreased reproductive performance if >15% of tested precalving cows have NEFA values > 0.30 mEq/L. Note, that as indicated above, pooling samples from individual cows is not recommended for herd-level testing.
- Postpartum NEFAs: At the herd-level, there is a significantly increased risk of post-calving metabolic and infectious diseases, decreased milk production or decreased reproductive performance if >15-20% of tested postcalving cows have NEFA values > 0.70 mEq/L. Note, that as indicated above, pooling samples from individual cows is not recommended for herd-level testing (Ospina et al 2010).
- Cow level testing
- Hepatic lipidosis in dairy cattle: In one study, a NEFA:cholesterol ratio >0.2 (SI units) had a 9.9 odds ratio of an animal having hepatic lipidosis (defined as >10% lipid content in the liver), with this cut-off being more sensitive (63% with 85% specificity) than BHB, NEFA alone or AST activity (>120 U/L) for diagnosis of hepatic lipidosis (based on area under the receiver operating characteristic curve or AUC). However, the animals in this study had blood measurements done at various times after lactation (Mostafavi et al 2013).
- Negative energy balance in small animals: This occurs primarily in diabetes mellitus. In a state of insulin deficiency or insulin resistance inhibition of hormone-sensitive lipase is lost. A regulatory imbalance of hormone-sensive lipase activity increases fat mobilization from adipose tissue to hepatic tissue, elevating the concentration of NEFAs.
- Negative energy balance in camelids: Sick, stressed camelids, particularly pregnant females, are at risk of developing hepatic lipidosis. Measurement of NEFA can be done to determine if they are in negative energy balance. Camelids with NEFA > 0.8 mEq/L (Tornquist et al 2001) are at increased risk for lipidosis. Anorexic camelids frequently have NEFAs that are increased but below this value and they may not develop lipidosis.
