Hemoglobin (Hgb), which consists of a heme group (porphyrin ring containing ferrous or Fe2+ iron) plus a pair of α and a pair of β globin chains, carries oxygen. In humans, hemoglobin is typically used to evaluate red blood cell mass versus PCV or HCT. However, in animals we generally default to the HCT or PCV because the hemoglobin can be falsely high in post-prandial samples from lipemia (see below), which we see more frequently than we would like in samples from dogs and cats.
Since red blood cells are approximately 33% hemoglobin, the hemoglobin concentration of whole blood normally is about one third of the HCT (i.e., the MCHC is 33 g/dL). This is true of most species, other than camelids, whose hemoglobin takes up just less than half of the RBC (MCHC around 40-45 g/dL).
Method of measurement
The ADVIA hematology analyzer provides two different measurement of hemoglobin concentrations of all the RBCs. One is a direct spectrophotometric measurement using cyanide and the other is an optical measurement using laser light (calculated or cellular hemoglobin) and only reflects intact RBCs. These usually provide similar results in normal animals but differing results in animals with abnormal blood features, e.g. lipemia, agglutination, hemolysis. We usually do not provide results for the calculated hemoglobin concentraiton, unless we think the hemoglobin results by the regular spectrophotometric method are inaccurate. We have provided a table in the MCHC/CHCM page for the different hemoglobin-related measurements that we can provide with our hemogram results (what they are, when we provide them).
-
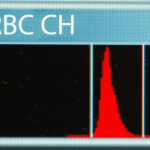
RBC CH histogram Spectrophotometric method using RBC lysis and cyanmethemoglobin (measured hemoglobin): Traditionally, the hemoglobin concentration is measured directly using the cyanmethemoglobin method after lysis of RBCs. A lysing agent is added to a sample of diluted blood; the lysing agent disrupts all the RBCs in the sample and releases the hemoglobin into the fluid so that the sample then consists of a solution of hemoglobin (or free hemoglobin). The hemoglobin is converted to a form called cyanmethemoglobin after addition of cyanide and the concentration is read by a spectrophotometer with the wavelength set at the peak absorbance of cyanmethemoglobin (around 540 nm). The concentration of hemoglobin is then measured from the absorption of the solution. Conditions which cause turbidity in the lysate used in this assay, such as lipemia, Heinz bodies, or free RBC nuclei (avian, reptilian blood) can result in falsely high absorbance and hence, overestimation of the hemoglobin concentration. Remember that hemoglobin-based oxygen carriers are red and are considered the equivalent of “free” hemoglobin so they will also be measured as hemoglobin with the cyanmethemoglobin method. This will result in high hemoglobin concentrations compared to the HCT and RBC count from the same patient. With this technique, if the RBC are already lysed in the blood or blood tube, the hemoglobin concentration will be the same as when all the RBCs are intact, i.e. it does not matter if the RBC were lysing in the patient with in vivo hemolysis, lysed in the tube with in vitro or artifactual hemolysis, or lysed in the machine by the added reagent. Thus, with in vitro hemolysis (an artifact of sample collection and handling), the measured hemoglobin concentration will be the most accurate result. With in vitro hemolysis, a HCT can be estimated from this hemoglobin measurement (by multiplying the hemoglobin x 3, because hemoglobin comprises approximately 1/3 of a RBC).
The measured hemoglobin is used to calculate the following RBC indices, which are routinely provided on hemogram results:- MCH (pg): This is equivalent to (Hgb ÷ RBC count) x 10. Thus, the MCH will be falsely increased if the Hgb concentration is falsely increased or the RBC count is falsely low (e.g. in vitro hemolysis).
- MCHC (g/dL): This is equivalent to (Hgb ÷ HCT or PCV) x 100. The MCHC will be affected by the same things that affect the MCH, with the addition of the MCV (since HCT = MCV x RBC count).
-
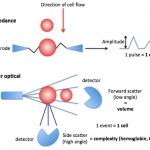
Laser-based hemoglobin analysis Light scatter or optically measured hemoglobin in intact RBCs (intracellular hemoglobin): The ADVIA hematology analyzer also measures the hemoglobin content within all RBCs directly, based on the internal complexity of the cells under laser light. The hemoglobin causes internal complexity which creates high angle light scatter or side scatter. Based on the degree of side scatter, the instrument “channelizes” the results, segregating the cells into channels representing relative ranges of hemoglobin content. This is illustrated as a frequency distribution curve or histogram (see image above). From the histogram, the analyzer obtains the average hemoglobin content within the intact RBCs (called CH) or the average hemoglobin concentration (called CHCM) of intact RBCs (the latter takes into account RBC volume whereas CH does not). These values can be more accurate than traditional methods of measuring hemoglobin using RBC lysis and cyanide, especially in conditions that falsely increase the latter hemoglobin concentration, such as hemoglobin-based oxygen carriers, lipemia, hemolysis or agglutination. The variation in hemoglobin content or concentration in intact RBCs can also be calculated as the hemoglobin distribution width (= standard deviation ÷ mean and expressed as a percentage), however this value is not reported on hemograms (for internal use only). The ADVIA also back-calculates a CHCM into a calculated or cellular hemoglobin concentration (g/dL), which takes into account the concentration of hemoglobin per unit volume of all the RBCs not just the mean concentration of the RBCs. We only use these results if we consider that the measured hemoglobin with the cyanide method is inaccurate (see above and table in the MCHC page).
- CH: Directly measured by high angle light scatter in the analyzer and is a measure of the amount (pg) of hemoglobin in each intact red blood cell (RBC must be intact to scatter the laser light, so prelysed RBC will not be detected with this method). A frequency distribution curve (histogram) of the variation in the hemoglobin content (CH) of intact RBC is also provided (see image above) and the mean CH is calculated from the histogram.
- CHCM: Directly measured by the analyzer, like the CH, but takes into account RBC volume so it is a concentration (g/dL). Like the CH, a frequency distribution curve is provided for the concentration and the mean concentration is calculated from the histogram.
- Calculated hemoglobin: The ADVIA back-calculates a hemoglobin (calculated hemoglobin) from the CHCM (i.e. calculated hemoglobin = (CHCM x MCV x RBC count) ÷ 1000. This provides reasonably accurate measurements of the hemoglobin concentration in conditions which falsely increase the hemoglobin by the cyanmethemoglobin method (usually lipemia), however values will be falsely low with in vitro hemolysis (only the hemoglobin content in unlysed RBC will be measured).
- Free hemoglobin refers to the difference between the calculated and measured hemoglobin. The difference between these two measurements is normally quite small unless there is lipemia or hemolysis.
Units of measurement
The regular lysis hemoglobin or calculated hemoglobin are expressed as g/dL of the blood (SI units are g/L). The conversion formula to SI units is as follows
g/dL x 10 = g/L
Sample considerations
Sample type
Whole blood
Anticoagulant
EDTA is the preferred anticoagulant. Although citrate can be used, the volume of citrate in the tube (10% of the collection volume) will dilute the measured hemoglobin (but not the calculated hemoglobin) accordingly. Heparinized whole blood can also be used.
Stability
Hemoglobin is quite stable (the most stable of all RBC results).
Interferences
- Lipemia: Will falsely increase measured hemoglobin and related calculated indices, MCH and MCHC.
- Hemolysis: Will decrease the calculated hemoglobin, CH and CHCM but has no effect on the measured hemoglobin. Hemolysis will also falsely increase the MCH (hemoglobin will be higher than RBC count) and MCHC (hemoglobin will be higher than HCT/PCV). In an animal with in vitro hemolysis versus true intravascular hemolysis, the measured hemoglobin is the best or most accurate estimate of the oxygen-carrying capacity of blood (multiply by 3 to obtain an approximate HCT). In contrast, the hematocrit (PCV), RBC count or calculated hemoglobin are a better estimate of the oxygen-carrying capacity of blood in an animal with true in vivo intravascular hemolysis (because the free hemoglobin in the animal’s plasma from the intravascular hemolysis cannot carry oxygen, but is measured as hemoglobin by analyzers).
- Icterus: No effect on either hemoglobin measurement.
- Other: Heinz bodies (many, particularly if large) may falsely increase the measured hemoglobin (Dondi et al 2019). Oxyglobin will contribute to (falsely increase) the measured hemoglobin concentration.
Test interpretation
Increased concentration
- Artifact:
- Measured hemoglobin: Lipemia (most common), Heinz bodies (typically if affects >50% RBCs in cats), oxyhemoglobin, RBC nuclei (many nRBC).
- Physiologic: Some breeds of dogs can have higher hemoglobin than others (for more see HCT).
- Pathophysiologic
- Relative change in RBC number to blood water: Dehydration, splenic contraction secondary to epinephrine (horses).
- Absolute increase in RBC mass (erythrocytosis): Stimulated by erythropoietin (secondary erythrocytosis) or erythropoietin-independent (primary erythrocytosis, e.g. polycythemia vera or chronic erythroid leukemia)
Decreased concentration
- Artifact:
- Calculated hemoglobin: Hemolysis of RBC due to sample collection or storage (in vitro hemolysis).
- Pathophysiologic
- Relative change of RBC to blood water: Over-dilution with fluids, splenic relaxation (anesthetic agents, tranquilizers).
- Absolute decrease in RBC mass: Indicates a true anemia, due to hemorrhage, hemolysis (intravascular, extravascular) or decreased production. Multiple mechanisms may be operative.
