Platelet numbers are interpreted with respect to the reference interval for that species. If reference intervals are not available, then we use the literature for guidelines on interpreting platelet counts. A decrease in platelet numbers (below the lower reference limit) is called thrombocytopenia, whereas an increase in platelet numbers (above the upper reference limit) is called a thrombocytosis. Thrombocytopenia is clinically more important than thrombocytosis because thrombocytopenia is associated with excessive hemorrhage. Guidelines as to the association between platelet count and hemorrhagic risk are given below. Thrombocytosis is usually asymptomatic (not associated with excessive hemorrhage or thrombosis in animals). Note that platelets are also important for vascular integrity, physically sealing minute defects and releasing growth factors that maintain normal barrier function (see Nachman and Rafii review, 2008) under physiologic and pathologic conditions.
| Platelet count (x 103/μL) | At risk of hemorrhage* | Subjective assessment of platelet numbers** |
| <10 | Yes (highest risk), usually spontaneous; induced by trauma or surgery | Marked decrease (very low) |
| <30 | Yes, can be spontaneous, induced by trauma or surgery | Marked decrease (very low) |
| 30-50 | Yes, usually secondary to trauma or surgery; can be spontaneous if a concurrent platelet function defect | Moderate decrease (low) |
| 50-100** | No, unless concurrent platelet function defect | Moderate decrease (low) |
| >100** | No, unless concurrent platelet function defect | Mild decrease (low) |
| * Not all animals with low platelet counts will show signs of hemorrhage. Guidelines based on human medicine (Jinna and Khandar, accessed 2021; for guidelines on transfusion requirements, see NICE). There is some thought that hemorrhage will only occur in thrombocytopenic animals if there is concurrent inflammation (Goerge et al 2008). ** This is species dependent and applicable to dogs, cats, ruminants and camelids. A platelet count of 100 x 103/μL is normal for a horse and would not be considered a thrombocytopenia. |
||
Thrombocytopenia
Thrombocytopenia can be inherited or acquired. Inherited or congenital causes of thrombocytopenia have been identified in dogs, as indicated below. Since inherited thrombocytopenia is not usually severe in affected breeds, a severe reduction in platelet count should raise suspicion of a pathologic cause, particularly in a bleeding animal.
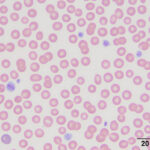
Inherited thrombocytopenia
- Cyclic Hematopoiesis of Gray Collies: Cyclic hematopoiesis of Gray Collies is an autosomal recessive disorder in humans and Gray Collie dogs characterized by cyclic fluctuations in the number of circulating neutrophils, reticulocytes, and platelets. The peripheral cytopenias are the result of a defect in hematopoietic stem cells. The neutropenic and thrombocytopenic episodes occur approximately every 12 days. Mortality is high in affected puppies and most die of infection before six months of age (DiGiacomo et al 1983). The hematopoietic defect can be corrected by bone marrow transplantation. The defect is caused by abnormalities in transport of the enzyme elastase in neutrophils due to a mutation in a subunit of an adaptor protein (AP-3) that facilitates protein sorting (Meng et al 2010).
- Inherited macrothrombocytopenia: Inherited macrothrombocytopenia, due to a mutation in β1-tubulin, has been documented in several dog breeds, notably the Cavalier King Charles Spaniel (CKCS), Norfolk and Cairn Terriers, but also other breeds (e.g. Labrador Retrievers, Poodle, Chihuahua, Shih Tzu, Maltese Terrier, Jack Russell Terriers). The tubulin mutation (which differs, depending on the breed) results in defective fragmentation of the megakaryocyte cytoplasm, producing decreased numbers of large platelets. In CKCS and Norfolk Terriers, platelet counts with impedance-or laser-based or manual methods range from as low as 30 to 150,000/µL and 19 to 110,000/µL, respectively, but affected dogs are usually asymptomatic and have normal platelet crits (due to increased platelet size). Affected dogs appear to be homozygous for the genetic defect, with heterozygotes having normal platelet counts (Gelain et al 2014, Davis et al 2008). Other breeds of dogs may suffer from an inherited macrothrombocytopenia with abnormally shaped platelets, such as Akitas (Hayakawa et al 2016, Caldin et al 2016).
- Breed-associated thrombocytopenia: Greyhounds and other sight hounds (e.g. whippets) can have lower platelet counts than other dog breeds (ranging from 80 to 295 x 10,000/µL), for reasons unknown.
Acquired thrombocytopenia
Acquired thrombocytopenia, usually immune-mediated, is the most common hemostatic disorder encountered in private veterinary practice. A platelet count should be performed on every animal presenting with clinical signs of hemorrhage. Thrombocytopenia can be an artifact, iatrogenic (dilutional) or due to pathophysiologic causes, which affect the bone marrow (decreasing production) or peripheral survival (consumption/usage, destruction/clearance, sequestration), as listed below. Naturally, artifact must be considered each time we get a platelet count, which is why we examine the feathered edge and body of the smear for platelet clumps. Iatrogenic causes should be considered if the animal is being given substantial amount of fluid therapy.
The main pathophysiologic mechanisms we think about in sick animals that can result in acquired thrombocytopenia are: 1) Decreased production, 2) Increased consumption/use (think clotting), 3) Increased clearance (usually immune-mediated destruction), and 4) Sequestration. In many diseases, there are multifactorial mechanisms for the thrombocytopenia. The degree of thrombocytopenia can be helpful in differentiating between mechanisms and potential causes (see thrombocytopenia table under interpretation summary).
Artifact/Iatrogenic mechanisms
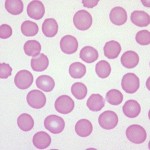
- Artifact: Consequence of platelet clumping. Thrombocytopenia can be of any degree.
- Dilutional: Marked hemodilution can cause a mild to moderate thrombocytopenia.
Pathophysiologic mechanisms (see more below)
- Decreased production of platelets in the marrow. Thrombocytopenia can be of any degree, usually moderate to marked.
- Increased consumption/use of platelets in coagulation. Thrombocytopenia can be of any degree, but is usually mild to moderate.
- Disseminated intravascular coagulation: This is the most common condition associated with increased consumption or use of platelets. The thrombocytopenia results because platelets are “consumed” with excessive activation of hemostasis.
- Acute severe to massive hemorrhage. Although this has been attributed to “loss” of platelets with blood, the thrombocytopenia seen in some animals with acute severe to massive blood loss is likely due to the cause of the blood loss or activation of coagulation with consumption of platelets. It is listed as a separate mechanism in some texts. The associated thrombocytopenia is usually mild and sometimes moderate. Note that hemorrhage needs to be acute (so the bone marrow has no time to respond) and severe to result in thrombocytopenia through this postulated mechanism. Mild to moderate and even, in many cases, localized severe hemorrhage will not result in a thrombocytopenia (we have more than enough platelets to do their job).
- Increased destruction/clearance of platelets by macrophages in the spleen and liver. Hepatocytes can also clear platelets directly if they are desialylated via the Ashwell-Morell receptor (Li et al 2015), although Kupffer cells in the liver also remove desialylated platelets (Depperman et al 2020). Thrombocytopenia can be of any degree, usually moderate to marked particularly in immune-mediated causes.
- Sequestration of platelets in the spleen (splenomegaly) or microvasculature (e.g. endotoxemia – platelets sequester in lungs transiently). Thrombocytopenia is usually mild. In one study of horses given 500 ng/kg lipopolysaccharide as an IV infusion, the platelet count was mildly to moderately decreased (mean nadir, 77,000/uL, with a standard deviation of about 50,000/uL, ranges not given) between 2-4 hours after infusion and after a brief rebound decreased mildly between 10-78 hours. The rapid decrease was associated with a marked leukopenia (all cell lineages decreased, however neutrophils and monocytes decreased severely within 1 hour post infusion) (Lillehöök et al 2020).
Artifactual thrombocytopenia will not elicit a bone marrow response, whereas the other peripheral causes of thrombocytopenia should elicit a bone marrow response (megakaryocytic hyperplasia) if the thrombocytopenia is of sufficient duration and severe enough. This response can be difficult to detect, however we can evaluate a bone marrow response to thrombocytopenia in the ways indicated below. Note, that these tests are not 100% sensitive or specific and usually we attempt to find the cause of the thrombocytopenia or evaluate response to therapy before embarking on additional costly testing.
- Large platelets (macroplatelets): Just like immature RBC are usually larger than mature RBC, young platelets can be (but are not always) larger than immature platelets (Handtke and Thiele review 2020). These large platelets are best visualized on a smear examination of blood versus using the electronic measurement of mean platelet volume (MPV). This is because the MPV is a mean of all platelet volumes (just like the mean corpuscular volume or MCV is a mean volume of RBC) and low numbers of large platelets may not increase the MPV above the reference interval. The presence of large platelets in a thrombocytopenic animal suggests the bone marrow is responding to the thrombocytopenia, however the absence of large platelets in a thrombocytopenic animal does not mean that the thrombocytopenia is definitely due to decreased marrow production for these reasons: a) Large platelets can be difficult to conclusively identify visually in a blood smear because there is a range of platelet size in normal animals, with some healthy animals having quite large platelets (which may not necessarily be young platelets), particularly cats; b) Large platelets may be present due to abnormal platelet production (see inherited macrothrombocytopenia above) or swollen platelets (with storage) versus immature platelets (just like macrocytic RBC can be due to abnormal RBC production or RBC swelling with storage). Note the mean platelet volume may be increased if there are sufficient large platelets in blood, but is also falsely increased by platelet clumping (a platelet clump is “seen” by the analyzer as one large platelet).
- Reticulated platelets: Just like we measure and can quantify immature anucleate RBC by detecting RNA in their cytoplasm, RNA in sufficient amounts can be detected in immature platelets. This requires detection of RNA in platelets using fluorescent dyes (e.g. thiazole orange), which bind to RNA, and specific hematologic analyzers (Pancraz et al 2009, Oellers et al 2016), most of which are not in current use in veterinary laboratories, or flow cytometry (Pancraz et al 2009, Wilkerson et al 2001) and is quite expensive. The results can be reported as a percentage or an absolute count (by multiplying the percentage by the absolute count) and can be falsely increased with storage of blood (Smith and Thomas 2002), although there is conflicting data on the latter (Wilkerson et al 2001). This is not a routinely performed test and usually not reported on routine hemograms. Studies have shown that thrombocytopenic dogs can have high numbers of reticulated or immature platelets containing RNA (Wilkerson et al 2001), including those with inherited disorders (Zmigrodzka et al 2014). Although a normal reticulated platelet count (% or absolute) in a thrombocytopenic animal would argue for a platelet production defect in marrow, in reality, this may not be the case (bone marrow production may be occurring). The presence of increased numbers of reticulated platelets would support a peripheral cause for the thrombocytopenia (consumption, destruction, sequestration, loss) but does not differentiate between causes of the thrombocytopenia (e.g. primary or secondary immune-mediated) (Wilkerson et al 2001).
- Megakaryocyte numbers in marrow: This is a subjective assessment and requires the presence of bone marrow spicules (stroma) in the aspirate. Numbers of megakaryocytes per spicule vary depending on method of smear preparation and species, and varies quite a bit within species (increasing subjectivity of the interpretation) (Mylonakis et al 2005). Megakaryocyte numbers are always interpreted with respect to the peripheral platelet count. A megakaryocytic hyperplasia is an expected response to a peripheral thrombocytopenia of duration and severity to stimulate thrombopoietin production by the liver (this is usually due to consumption or destruction). The absence of a megakaryocytic hyperplasia, particularly in an animal with moderate to severe thrombocytopenia, suggests (but does not necessarily indicate) that the thrombocytopenia is due to decreased marrow production. However, megakaryocytes may look normal and be in sufficient numbers and production or release may still be abnormal (cannot rule out a production defect even in the setting of megakaryocyte hyperplasia or normal megakaryocyte numbers in marrow). Note that a bone marrow aspirate is usually not done to assess if a thrombocytopenia is due to decreased production, unless there are other cytopenias (neutropenia, anemia) indicating generalized bone marrow disease. A bone marrow aspirate is usually reserved for those cases of isolated thrombocytopenia that are unresponsive to appropriate therapy.
Causes of acquired thrombocytopenia (a mechanistic approach)
Artifact or iatrogenic causes
- Artifact: Some analyzers cannot detect large platelets, particularly in healthy (see inherited macrothrombocytopenia above)or sick dogs with large platelets. Therefore, any provided platelet count (from any counting method) should be verified by an estimate from a smear (the platelet count estimate will be higher than the platelet count if the machine is “missing” or not counting large platelets).
- Platelet activation with collection: Platelet clumping in blood samples (as evident in peripheral blood smears) will falsely decrease the platelet count regardless of counting method, and in some instances, may result in a pseudothrombocytopenia. This is usually a consequence of difficult venipuncture, but may also occur in blood collected cleanly from sick animals (in these cases, it may indicate hyperactivation of platelets in vivo). Platelets of cats, in particular, clump readily with sample collection making it difficult to obtain an accurate count in this species.
- EDTA-dependent thrombocytopenia: There have been rare reports in humans, with isolated reports in horses (Hinchcliff et al 1993) and dogs (Wills and Wardrop 2008), of an EDTA-dependent pseudothrombocytopenia. This only occurs in blood collected into EDTA (not citrate or heparin) due to EDTA-induced unmasking of platelet antigens by EDTA with binding of natural antibodies and subsequent platelet aggregation occurring in the blood tube. In these circumstances, platelet counts do need to be determined from citrated or heparinized blood samples (although citrate is preferred as heparin can also produce platelet clumping). However, this is rare occurrence and samples for platelet count testing should be collected into EDTA and not citrate or heparin on a routine basis.
- Dilutional: Excessive dilution with administration of platelet-poor products, e.g. plasma, stored blood, can result in a mild to moderate thrombocytopenia. Since a dilutional thrombocytopenia may be transient, a bone marrow response (megakaryocytic hyperplasia) may not be stimulated, but if persistent and low enough, a megakaryocytic response would occur (as long as the bone marrow is not suppressed due to other reasons).
Pathophysiologic causes
- Decreased platelet production: Platelets are produced by megakaryocytes in the bone marrow and are actually fragments of the megakaryocyte cytoplasm. The normal lifespan of platelets in dogs (and presumably other species) is around 5-7 days (Tanaka et al 2002). Megakaryocyte production of platelets could be abnormal as a consequence of general bone marrow disease (e.g. leukemia, bone marrow necrosis, bone marrow aplasia, infiltrative neoplasia), in which case, other cell lineages could be affected, specifically neutrophils (which have a short lifespan in blood of around 12-24 hours) and red blood cells (these have a longer lifespan of around 60 days in cats and 120 days in dogs). General bone marrow disease usually results in bi- or pancytopenia in peripheral blood, therefore decreased production of platelets is the most likely mechanism for a thrombocytopenia in an animal with other cytopenias (specifically neutropenia, non-regenerative anemia). The absence of reticulated platelets would also suggest bone marrow production of platelets is problematic in a thrombocytopenic animal, however the absence of large platelets gives us no indication of bone marrow production capabilities. We can definitively attribute a thrombocytopenia to decreased platelet production when there are no or decreased numbers of megakaryocytes in a bone marrow aspirate or the absence of immature (reticulated) platelets in peripheral blood. However, as indicated above a bone marrow aspirate is not routinely performed in any thrombocytopenic animal. In addition, the presence of megakaryocytes, even in increased numbers, does not necessarily mean platelets are being produced or released into the circulation, thus decreased production cannot be ruled out even if there was a megakaryocytic hyperplasia in a bone marrow aspirate. Megakaryocyte production of platelets is usually decreased as part of generalized bone marrow disease, however a selective thrombocytopenia due to decreased production can be seen under the following situations:
- Infectious agents: Viruses can directly infect megakaryocytes, causing cell death or decreased platelet production, e.g. bovine virus diarrhea, canine distemper. Viruses can also induce the production of cytokines that inhibit thrombopoiesis. Thrombocytopenia in some bacterial infections, e.g. Ehrlichia canis, has been attributed, in some cases, to decreased marrow production of platelets (which could be due to immune-mediated effects).
- Immune-mediated: Immune-mediated destruction or inhibition of megakaryocytes in bone marrow can result in severe thrombocytopenia. A syndrome of hemorrhage has been reported in calves, called bovine neonatal pancytopenia, which receive allo-antibodies from colostrum. The antibodies cause marrow injury to progenitors resulting in bone marrow aplasia and pancytopenia, which manifests clinically as hemorrhage from severe thrombocytopenia. The antibodies are produced in response to vaccination of the dams with a certain bovine diarrhea vaccine that contains bovine antigens, stimulating an immune response (Bell et al 2013, Euler et al 2013). Some dogs with immune-mediated thrombocytopenia have decreased or absent megakaryocytes in bone marrow, suggesting that the main mechanism of the thrombocytopenia in these cases is an immune-mediated attack against progenitors in marrow (megakaryocytes). However, this is an uncommon cause of thrombocytopenia in animals. Immune-mediated disease (primary or secondary to drugs, infectious agents, neoplasia) more usually results in thrombocytopenia from increased clearance of circulating platelets, not decreased platelet production.
- Neoplasia: In our experience, lymphoid neoplasia (T cell neoplasms such as acute lymphocytic leukemia and lymphoma of T cell lineage) can manifest as an isolated thrombocytopenia in dogs and horses. This could be due to immune-mediated (direct cytotoxic, antibody-mediated, cytokine-mediated) inhibition or destruction of megakaryocytes in marrow as well as increased clearance of circulating platelets.
- Drugs: Certain drugs can selectively affect bone marrow production of platelets. This can be due to immune-mediated or direct cytotoxic effects. For example, chemotherapeutic agents can affect megakaryocytes in the bone marrow, resulting in a predictable thrombocytopenia 7-10 days after treatment. Other drugs, such as trimethoprim-sulfur drugs, can cause an immune-mediated thrombocytopenia without other cytopenias.
- Increased platelet consumption/use: This generally refers to use of platelets during hemostasis (platelet plug formation), however pathogen- or damage-associated molecular patterns that induce platelet activation and/or aggregation could also result in thrombocytopenia via this mechanism. Normally, platelets are in excess so mild or even moderate blood vessel injury does not usually result in thrombocytopenia as a consequence of physiologic hemostasis. However, more severe or massive endothelial injury, particularly when acute, can result in a thrombocytopenia due to attempts at platelet plug formation withoμt an animal being in disseminated intravascular coagulation (DIC). Under most pathologic conditions, increased platelet consumption occurs due to excessive and abnormal platelet use in clotting, particularly with the syndrome of DIC. In the vast majority of situations, a severe thrombocytopenia is the cause of hemorrhage and not a consequence of the hemorrhage. The bone marrow would be expected to respond to this peripheral cause of thrombocytopenia by stimulating megakaryopoiesis (megakaryocytic hyperplasia), unless there is concurrent inhibition of the bone marrow response.
- Acute severe hemorrhage: Severe (massive), acute hemorrhage due to trauma or anticoagulant rodenticide toxicosis (Waddell et al 2013) can result in mild to moderate thrombocytopenia (usually not < 50,000/µL). This has been attributed to “loss” of platelets with blood as well as increased “use” in an attempt to stop the hemorrhage. The thrombocytopenia may also be occurring because the bone marrow has not yet had time to respond to the drop in platelet count. An experimental study of acute blood loss in dogs showed that there was no change in platelet count with loss of up to 40% blood volume (Lynch et al 2016). This study suggests that thrombocytopenia in animals with acute blood loss is likely secondary to the cause of the blood loss or initiation of coagulation than just a consequence of “loss of platelets” with blood loss.
- Platelet activation and aggregation: Certain bacteria, such as Staphylococcus, possess clumping factors, which promote platelet aggregation via binding to fibrinogen/fibronectin and low affinity GPIIb/IIIa on resting platelets. This could potentially result in thrombocytopenia in severe sepsis. Platelets also express toll-like receptors and these can also be involved in platelet aggregation mediated by infectious agents, such as bacteria (Depperman and Kubes review 2016).
- Increased platelet destruction or clearance: This can occur through various mechanisms, particularly immune-mediated clearance, but desialyation (Li et al 2015), apoptosis and activation can also result in increased clearance of platelets (e.g. activation can result in the formation of neutrophil- or monocyte-neutrophil complexes, which the leukocytes then phagocytizing the platelets). Causes of platelet activation include foreign agents (such as stents), vasculitis (e.g. secondary to Rocky Mountain Spotted Fever) or endocarditis, infectious agents (e.g. viruses [Assinger 2014]), and snake venoms, some of which can have platelet aggregatory effects.
- Immune thrombocytopenia (ITP): This used to be called immune-mediated thrombocytopenia, but is now called immune thrombocytopenia (LeVine and Brooks 2019). This can be a primary disorder, i.e. the clinical syndrome of idiopathic (primary) immune thrombocytopenia (ITP), or as a component of other infectious (e.g. Ehrlichia canis [Cortese et al 2011]), neoplastic or systemic immunologic diseases (e.g. systemic lupus erythematosis or SLE). Immune clearance is thought to be mostly mediated by antibodies, but can also be due to destruction of platelets via cytotoxic T cells (LeVine and Brooks 2019). Detection of IgG on platelets (by flow cytometric detection of antibodies bound to platelets in blood) or megakaryocytes (by direct immunofluorescence methods on bone marrow aspirates) of thrombocytopenic animals is considered as supportive evidence of antibodies with anti-platelet activity. However, these procedures are neither sensitive nor specific and does not discriminate between ITP or immune-mediated clearance of platelets secondary to another disease (e.g. Ehrlichiosis, neoplasia, SLE) or drugs (Wilkerson et al 2001). Increased clearance of platelets should stimulate a bone marrow response, unless the disorder is concurrently affecting bone marrow production of platelets (e.g. the immune-mediated response may be against megakaryocytes as well as platelets in circulation – megakaryocytes are thought to be damaged or destroyed by cytotoxic T cells [LeVine and Brooks 2019], although this has not been shown in dogs). There is also evidence in humans that thrombopoietin levels are not increased or insufficiently increased in ITP, leading to decreased thrombopoiesis. This is thought to be mediated by the decreased removal of desialyated platelets by the liver, which normally positively feeds back on erythropoietin production (more platelets are destroyed outside of the liver than normal, by macrophages in other sites, such as the spleen) (LeVine and Brooks 2019).
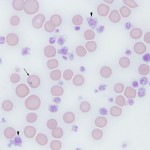
- Primary ITP: ITP is diagnosed most frequently in dogs but rare presumptive cases of ITP in cats (Garon et al 1999) and horses (Larsen et al 1983) have been reported. Examination of a peripheral blood smear in animals with ITP show very few or no platelets in severe cases (see image above). Primary ITP usually presents as a clinical syndrome of acute onset small vessel type bleeding in a dog without signs of severe systemic illness. The degree of anemia is variable and depends on the amount of blood lost through hemorrhage and the presence of concurrent immune-mediated destruction of red blood cells (hemolytic anemia). The diagnosis of ITP is based on finding thrombocytopenia, normal coagulation screening test results, exclusion of other diseases (including testing for infectious agents), and a favorable response to immunosuppressive therapy. Bone marrow aspiration is generally not indicated in the initial work-up of a patient with ITP; results usually show normal erythroid and granulocytic lines and, in most cases, normal to increased numbers of megakaryocytes. However, a bone marrow is performed if animals do not respond to immunosuppressive therapy as expected. Rare dogs with ITP have no or few megakaryocytes in marrow; such patients are considered to have immune-mediated destruction of megakaryocytes as well. These dogs are thought to respond more poorly to immunosuppressive therapy than dogs with megakaryocytic hyperplasia (Cooper et al 2016). Platelet destruction in some animals can be quickly controlled with fairly mild immunosuppression (corticosteroids alone) but in others prolonged treatment with cytotoxic drugs (e.g. azathioprine and/or vincristine in combination with corticosteroids) is necessary to increase the platelet count. Dogs in the latter category need intense supportive care to manage the side effects of the immunosuppressive drugs and the loss of blood. Since the spleen is considered the major site of platelet destruction, splenectomy is done in rare cases refractory to control by drugs. Because ITP can be a part of more generalized immune-mediated disease, clinical signs, physical findings, history, and laboratory results should be evaluated for evidence of abnormalities of other organs, particularly skin, joints, and kidneys, that may lead to recognition of SLE. In human patients with ITP, there is a trend by some clinicians towards observing patients that are not clinically bleeding when they have platelet counts >20-30 thousand/uL or normal quality of life. In contrast, those patients with platelet counts of <20,000/uL) or those with higher counts and evidence of hemorrhage are treated for the disorder (Cuker and Neunert 2016).
- Neonatal thrombocytopenic purpura: This occurs because of differences in platelet antigen expression in sires and dams, similar to neonatal isoerythrolysis. This has been recognized in pigs, mules and a Quarterhorse foal (Buechner-Maxwell et al 1997, Ramirez et al 1999, Forster 2007). In pigs, agglutinating antibodies against sire and piglet platelets have been documented in the dam. The piglets are generally born healthy, then develop thrombocytopenia after 5 to 9 days, with the nadir occurring at 10 to 13 days. Thrombocytopenia is due to increased platelet destruction and decreased platelet production. Clinical signs of cutaneous hemorrhage are seen. Death may result at 2 to 3 weeks of age. Surviving piglets appear clinically and hematologically normal by 16 weeks of age. Severe thrombocytopenia was discovered in a 1 day old Quarterhorse foal presenting for weakness and failure to suckle. The dam’s serum contained antibodies that bound to the foal’s and a full brother’s platelets (Buechner-Maxwell et al 1997). We have also similar cases in foals (equine and mule) at Cornel University.
- Secondary ITP occurs with infectious diseases, drugs, neoplasia and immune-mediated disorders.
- Infectious agents: Immune-mediated destruction of platelets is one of several possible mechanisms by which infectious diseases can cause thrombocytopenia. Thrombocytopenia is seen in some viral infections and may occur within 7-10 days of vaccination with modified-live viruses (especially canine distemper virus). This post-vaccinal thrombocytopenia is generally mild (platelet counts rarely decrease < 100,000/µL), with the most severe decreases seen in puppies. Immune-mediated destruction is partly responsible for the thrombocytopenia of canine ehrlichiosis (E. canis [Cortese et al 2011]) and infectious canine cyclic thrombocytopenia (Anaplasma platys). Thrombocytopenia is a common hematologic finding in horses with Anaplasma phagocytophilum infection. The severe thrombocytopenia in some cows with bovine virus diarrhea infection (BVD) appears to be due in part to antibody-mediated destruction. Infection with Babesia species, a protozoan erythroparasite, is frequently associated with thrombocytopenia. Similarly, Lyme disease (borreliosis) can be associated with thrombocytopenia, which may be due to immune-mediated mechanisms (the thrombocytopenia can be somewhat cyclical, varying from mild to moderate over time, in affected patients).
- Drugs: Many drugs have been reported to cause ITP in human patients and therefore are potential causes of thrombocytopenia in animals. In general, if an animal with the clinical syndrome of ITP is receiving drugs, these should be discontinued if at all possible and reintroduced with caution when a normal platelet count is restored. Documented cases of drug-related ITP in animals are few but include gold compounds, propylthiouracil and methimazole (anti-thyroid drugs used to treat some cats with hyperthyroidism), sulfonamides, and penicillin.
- Neoplasia: Immune-mediated thrombocytopenia is associated with neoplastic diseases in some animals. Neoplasms such as lymphoma and a variety of benign and malignant solid tumors have been implicated in some cases. Histiocytic sarcoma can also cause a thrombocytopenia due to increased clearance of platelets (not necessarily immune-mediated) by the neoplastic histiocytes.
- Transfusion-related: Immune-mediated thrombocytopenia due to blood transfusions (so-called post-transfusion purpura) is a rare condition in human patients characterized by a severe thrombocytopenia 1 to 2 weeks after blood or blood product transfusion. This has been reported in a dog with hemophilia A. The dog developed a severe thrombocytopenia (platelet count < 10,000/µL), on 2 occasions after being transfused (the first occurred 8 days after a second blood transfusion; the second, 5 days after cryoprecipitate infusions). On both occasions, the platelet counts normalized within 4 to 6 days of corticosteroid therapy (but the dog may have recovered spontaneously without treatment) (Wardrop et al 1997).
- Immune thrombocytopenia (ITP): This used to be called immune-mediated thrombocytopenia, but is now called immune thrombocytopenia (LeVine and Brooks 2019). This can be a primary disorder, i.e. the clinical syndrome of idiopathic (primary) immune thrombocytopenia (ITP), or as a component of other infectious (e.g. Ehrlichia canis [Cortese et al 2011]), neoplastic or systemic immunologic diseases (e.g. systemic lupus erythematosis or SLE). Immune clearance is thought to be mostly mediated by antibodies, but can also be due to destruction of platelets via cytotoxic T cells (LeVine and Brooks 2019). Detection of IgG on platelets (by flow cytometric detection of antibodies bound to platelets in blood) or megakaryocytes (by direct immunofluorescence methods on bone marrow aspirates) of thrombocytopenic animals is considered as supportive evidence of antibodies with anti-platelet activity. However, these procedures are neither sensitive nor specific and does not discriminate between ITP or immune-mediated clearance of platelets secondary to another disease (e.g. Ehrlichiosis, neoplasia, SLE) or drugs (Wilkerson et al 2001). Increased clearance of platelets should stimulate a bone marrow response, unless the disorder is concurrently affecting bone marrow production of platelets (e.g. the immune-mediated response may be against megakaryocytes as well as platelets in circulation – megakaryocytes are thought to be damaged or destroyed by cytotoxic T cells [LeVine and Brooks 2019], although this has not been shown in dogs). There is also evidence in humans that thrombopoietin levels are not increased or insufficiently increased in ITP, leading to decreased thrombopoiesis. This is thought to be mediated by the decreased removal of desialyated platelets by the liver, which normally positively feeds back on erythropoietin production (more platelets are destroyed outside of the liver than normal, by macrophages in other sites, such as the spleen) (LeVine and Brooks 2019).
- Increased platelet sequestration: The excessive platelet pooling in an enlarged spleen can lead to development of thrombocytopenia, which is usually transient. However, this is an uncommon cause of thrombocytopenia and platelet counts are generally only mildly decreased. Since this is usually transient and mild, megakaryopoiesis may not be substantially stimulated (may not see a megakaryocytic hyperplasia). Thrombocytopenia in animals with bacteremia or endotoxemia is due in part to sequestration of damaged platelets in organs such as lung, liver and spleen. In addition, LPS-mediated activation of platelets via binding to toll-like receptor 4 leads to platelet sequestration in the lungs in a neutrophil-dependent manner (likely through forming neutrophil-platelet aggregates) (Depperman and Kubes review 2016). However, animals with these diseases are likely to have DIC as well, which further contributes to the thrombocytopenia by consumption in excessive activation of hemostasis.
Thrombocytosis
Thrombocytosis may occur in primary myeloproliferative conditions or as a secondary (reactive) phenomenon in a variety of physiologic and pathologic states. Thrombocytosis is usually due to an increase in platelet production and release (in reactive cases, this is likely due to increased thrombopoietin or other inflammatory cytokines, that stimulate thrombopoiesis or thrombopoietin release, such as interleukin-6, CCL5), rather than an increased platelet lifespan. Usually, a platelet count higher than the reference interval for the species is a reactive thrombocytosis and not of direct pathologic importance. In our experience, young animals, in particular calves and foals, normally have platelet counts higher than the adult reference interval. Inherited causes of thrombocytosis have not been identified in animals.
- Drugs: Several drugs have been associated with a thrombocytosis, including corticosteroids and β-adrenergic drugs. The mechanism of corticosteroid-induced thrombocytosis is unclear. With epinephrine or norepinephrine, sympathetic stimulation has been shown to promote proplatelet production by megakaryocytes and facilitate recovery from bone marrow injury in mice (Chen et al 2016). A rebound thrombocytosis may be seen with recovery after bone marrow injury with antineoplastic agents. Some drugs, such as vincristine, are used to treat immune-mediated thrombocytopenia because they stimulate platelet production.
- Post-splenectomy: Due to its large vascular mass, the spleen contains up to 1/3 of the total platelet mass. The spleen is also a site or removal of effete or damaged platelets. Removal of the spleen can result in a transient thrombocytosis. In one study of 34 dogs in which a splenectomy was performed to remove splenic masses (benign in 50% and malignant in 50% of the dogs), the platelet count increased within 2 days of surgery from a mean ± SD of 168 ± 141 thou/uL to a peak at 7 days after surgery of 716 ± 332 thou/uL. The platelet counts then started to decrease but mean counts were still high at 14 days post surgery (582 ± 197 thou/uL) (Phipps et al 2020).
- Reactive thrombocytosis: Reactive thrombocytosis can be seen in a variety of disorders, including neoplasia, chronic inflammatory diseases, immune-mediated disease (immune-mediated hemolytic anemia, non-regenerative immune-mediated anemia), trauma (fractures, diaphragmatic hernia), and iron deficiency anemia. This is largely attributed to inflammatory cytokine release, such as interleukin-6, which stimulates thrombopoietin secretion or CCL5 (RANTES), which is released by platelets and stimulates megakaryopoiesis (Machlus et al 2016). Animals in the early stages of recovery from immune-mediated thrombocytopenia or prior bone marrow suppression may have transient thrombocytosis with platelet counts > 1 million/µL. In one retrospective study in 165 dogs, the most common disorders associated with a reactive thrombocytosis were neoplasia (especially lymphoma and mast cell tumors), inflammation particularly involving the gastrointestinal tract (especially pancreatitis, chronic hepatitis, and inflammatory bowel disease) and endocrine disorders (including diabetes mellitus, hyperadrenocorticism and hypothyroidism) (Neel et al 2012). The study was confounded by concurrent administration of drugs that can cause thrombocytosis (vincristine, corticosteroids). In cats, infectious or inflammatory disorders were the most common underlying diseases and the gastrointestinal system the most frequently involved organ (Rizzo et al 2007). In horses, the most common conditions were gastrointestinal tract disease (colitis), infectious or inflammatory disease (e.g. peritonitis, pleuritis) and miscellaneous causes, including hepatic disease, fractures and neoplasia (Sellon et al 1997).
- Neoplastic thrombocytosis: In rare cases, the cause of persistent thrombocytosis is essential thrombocythemia (chronic platelet leukemia), a chronic myeloid leukemia affecting megakaryocytes, resulting in extremely high platelet counts (usually > 1 million/µL). A bone marrow aspirate will reveal a megakaryocytic hyperplasia, therefore definitive diagnosis relies upon ruling out other diseases that may produce a thrombocytosis. If abnormalities are observed in megakaryocytes (dysplasia), this could be due to reactive or neoplastic causes, but these findings would be more expected in the latter. A concurrent basophilia is some human patients with this neoplasm and in dogs (Favier et al 2004). Essential thrombocythemia has been documented rarely in both dogs and cats (Hopper et al 1989, Hammer et al 1990, Bass and Schultz 1994). In human beings, essential thrombocythemia requires 4 major criteria or 3 major criteria plus one minor criteria, with the major criteria being: a) Documentation of a persistent thrombocytosis (>450,000/μL) on more than 3 occasions over at least a 3 months; b) Megakaryocytic hyperplasia with minimal fibrosis in the bone marrow; c) Not meeting criteria of other chronic myeloid leukemias; and d) Presence of mutations associated with essenthial thrombocythemia, e.g. JAK2, MPL). Minor criteria include a) Ruling out other causes of thrombocytosis (such as underlying iron deficiency) and b) Presence of a clonal marker (Arber et al 2016). Similar criteria, specifically major criteria “a” and minor criteria “b” (the barest minimum available to us as veterinarians since we lack the availability of mutation testing) have not been applied to these case reports, therefore many of them could have been a reactive thrombocytosis. Occasionally, thrombocytosis may also accompany other forms of chronic myeloid leukemia, such as polycythemia vera (chronic erythroid leukemia).
Related links
- Genetic testing for β1 tubulin defects in dog breeds.
