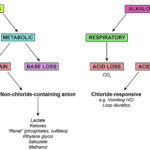
The different types of acid-base disturbances are differentiated based on:
- Origin: Respiratory or metabolic
- Primary or secondary (compensatory)
- Uncomplicated or mixed: A simple or uncomplicated disturbance is a single or primary acid-base disturbance with or without compensation. A mixed disturbance is more than one primary disturbance (not a primary with an expected compensatory response).
Acid-base disturbances have profound effects on the body. Acidemia results in arrhythmia, decreased cardiac output, depression, and bone demineralization. Alkalemia results in tetany and convulsions, weakness, polydipsia and polyuria. Thus, the body will immediately respond to changes in pH or H+, which must be kept within strict defined limits. As soon as there is a metabolic or respiratory acid-base disturbance, body buffers immediately soak up the proton (in acidosis) or release protons (alkalosis) to offset the changes in H+ (i.e. the body compensates for the changes in H+). This is very effective so minimal changes in pH occur if the body is keeping up or the acid-base abnormality is mild. However, once buffers are overwhelmed, the pH will change and kick in stronger compensatory responses. Remember that the goal of the body is to keep hydrogen (which dictates pH) within strict defined limits.
The kidney and lungs are crucial for allowing the body to respond to an acid-base disturbance and for maintaining normal acid-base balance. Of course, an acid-base disturbance can be the consequence if things go wrong with these organs (but is not an inevitable consequence of lung or renal disease – it all depends on the disease).
- Lungs: The lungs compensate for a primary metabolic condition and will fix theprimary respiratory disturbance if the disease or condition causing the disturbance is resolved.
The lungs:- Blow off carbon dioxide (it is an acid – carbonic acid): CO2 is more diffusible across membranes than O2 (so it is easier to blow off).
- Retain and are the source of oxygen.
- Kidney: The kidney is responsible for compensating for a primary respiratory disturbance or correcting (or attempting to correct for) a primary metabolic disturbance. Thus, normal renal function is essential for the body to be able to adequately neutralize acid-base abnormalities and will help return or correct the pH (or H+) to normal, once the primary disorder has ceased. Note that mild renal disease or dysfunction or a prerenal azotemia due to mild hypovolemia should not adversely impact the ability of the kidney to respond to an acid-base disturbance. Since the kidney is so crucial for normal acid-base balance, renal disease can result in acid-base abnormalities (usually acidosis, due to failure to excrete the normal acid load generated by protein metabolism). This occurs particularly with acute kidney injury, but also chronic renal disease leading to failure.
The kidney:- Maintains normal acid-base balance: Excretes the daily acid load (called titratable acidity) from protein metabolism.
- Compensates for a primary respiratory disorder and attempts to correct for a primary metabolic disorder (acidemia or alkalemia).
- With acidemia, the kidneys gets rid of acids as follows:
- Simple excretion via glomerular filtration (non-chloride non-volatile acids, e.g. ketoacids).
- Active excretion of protons (pH of urine expected to go down):
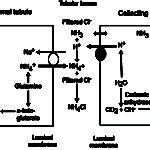
- NH4Cl via increased ammoniagenesis in the proximal convoluted tubules. The hydrogen is excreted with ammonium chloride in both proximal and distal tubules.
- H+ excretion via a vacuolar-type (V) ATP-dependent antiporter (V-H+-ATPase) in type A intercalated cells in the late distal and collecting tubules (Cl goes along for the ride via a paracellular pathway, partly due to electrochemical gradients, i.e. increased positive charge in tubular lumen). In the cortical collecting tubule, this transporter is dependent on sodium resorption by principal cells (which creates a lumen negative potential, facilitating proton secretion) and aldosterone, whereas in the medullary collecting tubule, this transporter is sodium independent (but also influenced by aldosterone).
- Active hydrogen excretion is linked to bicarbonate absorption so…..the net effect of the kidney’s active excretion of protons is: Acid (H+) loss with chloride (in excess of sodium) = bicarbonate (base) gain (metabolic alkalosis).
- With alkalemia, the kidney gets rid of base (HCO3–) as follows:
- Simple excretion via glomerular filtration.
- Active excretion of bicarbonate into the lumen by type B intercalated cells in collecting tubules (pH of urine expected to go up). This is linked to chloride resorption via a chloride/bicarbonate transporter (pendrin), which moves from the basolateral to the apical (luminal) surface.
- Decreased acid excretion
- Reduced ammoniagenesis (NH4Cl is retained) in proximal convoluted tubules.
- Reduced H+-ATPase activity in the late distal and collecting tubules (chloride is passively retained).
- Net effect: Gain of chloride-containing acid (hyperchloremic metabolic acidosis) = loss of bicarbonate.
- With acidemia, the kidneys gets rid of acids as follows:
FYI: In a primary metabolic disturbance, we do not expect the attempted correction of the kidney to normalize the pH unless the primary disturbance has been lessened by our intervention or has ceased so the pH is not continued to be driven down or up (in an acidosis or alkalosis, respectively). So once the primary disturbance is gone, the kidney will rapidly correct the change in pH (unless we did so first) if renal function is normal. If the primary disturbance is still ongoing and driving the pH down or up, an attempted corrective renal response (which requires normal and ideally optimal renal function) is not expected to return the pH to normal. In addition, in a primary metabolic alkalosis, the kidneys attempt to correct the pH increase is compromised and the kidney actually worsens and not lessens the alkalosis (see below for more detail).
There are four primary types of acid-base disorders, which the body responds to.
- Metabolic acidosis: This is due to increases in non-volatile (non-carbonic) acids, which can contain chloride as their anion (e.g. ammonium chloride, NH4Cl) or another anion, which does not contain chloride (e.g. lactate). The following body buffers try and offset the increase in non-carbonic acid:
- Bicarbonate in plasma and interstitial fluid buffers around 40% of the acid load. This will drop the bicarbonate concentration
- If the acid contains chloride (e.g. HCl, NH4Cl), you will see a hyperchloremic acidosis, i.e. low bicarbonate and high corrected chloride
- If the acid does not contain chloride and is an unmeasured anion (e.g. lactate), you will see a high anion gap acidosis, i.e. low bicarbonate and high anion gap
- Plasma proteins with negative charges on amino acids, such as albumin, buffer about 1% of the acid load, by accepting protons (H+).
- The rest of the acid load is buffered by intracellular buffers
- Hemoglobin in red blood cells.
- Phosphate-containing proteins in cells and bone (calcium moves into plasma in exchange for the intracellular movement of hydrogen, so a chronic acidosis results in dimineralization of bone).
- Body buffers in plasma (bicarbonate particularly, but also proteins) and intracellularly (hemoglobin in RBCs in particular) or in bone immediately start to offset any increase in H+ from a non-volatile acidosis.
- The lungs also blow off carbon dioxide, which is respiratory compensation.
- If needed, the kidney will kick in and increase ammoniagenesis (regenerating new bicarbonate and excreting ammonium chloride or NH4Cl in the proximal tubules and ascending limb of the loop of Henle; see image above) and excreting H+ directly via the V-H-ATPases (in all tubules, but primarily collecting tubules) as attempted correction for the acidosis (as long as the kidney is not dysfunctional and causing the acidosis in the first place).
- Bicarbonate in plasma and interstitial fluid buffers around 40% of the acid load. This will drop the bicarbonate concentration
- Respiratory acidosis: This is due to increases in the volatile (it can be blown off) or so-called “respiratory” acid, carbonic acid, which comes from increases in carbon dioxide due to inadequate ventilation.
- Carbon dioxide is freely diffusible and moves rapidly into cells (RBCs in particular).
- Intracellular buffers (hemoglobin in RBCs) bind the protons o offset any increase in H+ from a volatile acidosis. The generated bicarbonate gets moved into plasma in exchange for chloride via a bicarbonate/chloride exchanger.
- The kidney will kick in and increase ammoniagenesis (regenerating new bicarbonate and excreting ammonium chloride or NH4Cl) and excreting H+ directly via V-H-ATPases (chloride follows) as compensation for the volatile acidosis (this is very effective. In some species, the renal compensatory response to a primary respiratory acidosis may normalize the pH given time, which is usually weeks.
- Carbonic acid, which generates bicarbonate when combined with water, cannot (obviously) be buffered by bicarbonate.
- Carbon dioxide is freely diffusible and moves rapidly into cells (RBCs in particular).
- Metabolic alkalosis: This is due to accumulation of a base or loss of a non-volatile acid (which usually but does not always contains chloride as its anion).
- Body buffers in serum (proteins) and intracellularly (hemoglobin in RBCs in particular) immediately start to offset any decrease in H+ from a metabolic alkalosis by releasing protons.
- The lungs also retain carbon dioxide, which is respiratory compensation.
- If needed, the kidney will kick in and decrease ammoniagenesis (thus reducing ammonium chloride or NH4Cl excretion and bicarbonate generation, thus retaining H+ and chloride) and decreasing activity of the V-H-ATPases (retaining H+ and chloride) as attempted correction for the alkalosis (as long as the kidney is not dysfunctional and causing the acidosis in the first place). The kidney also filters excess bicarbonate in plasma and can actively excrete bicarbonate via type B intercalated cells in collecting tubules. However, the renal response to a primary metabolic alkalosis is frequently compromised and worsens the alkalosis versus reducing it.
- Respiratory alkalosis: This is due to decreases in carbon dioxide or carbonic acid secondary to hyperventilation (increased tidal volume). The body responds to a decrease in pCO2 as follows:
- Hydrogen is released off intracellular buffers (hemoglobin in RBCs in particular), which moves extracellularly offsetting the decrease in H+ in plasma (bicarbonate moves into cells, in exchange for chloride which moves into plasma)
- The kidney will kick in and decrease ammoniagenesis and H+ excretion (chloride is retained). This is very effective and, in some species, this metabolic compensatory response to a primary respiratory alkalosis can normalize the pH given time (weeks).
Combinations of these primary disturbances (more than one primary at the same time) results in a mixed disturbance. Note, that you cannot have a primary respiratory acidosis and a primary respiratory alkalosis at the same time; the lungs can create only one primary disturbance. You can also NOT have a primary respiratory disorder and a compensatory respiratory response at the same time. But you can have a primary metabolic acidosis (e.g. accumulation of lactic acid) and a primary metabolic alkalosis (vomiting gastric HCl) at the same time.
In general, primary disturbances can be distinguished from secondary or compensatory responses by the pH and degree and direction of change of the acid-base results. For example, an acidemia indicates that there is an acidosis and it is the dominant disturbance. If the bicarbonate and base excess are low, it indicates a primary metabolic acidosis. If the pCO2 is high, it indicates a primary respiratory acidosis. If the bicarbonate and base excess are low and the pCO2 is high, it indicates a mixed primary metabolic acidosis (low bicarbonate or base excess) and primary respiratory acidosis (high pCO2). In the latter scenario, the pH would be expected to be quite low (very acidemic), because of the combination of two primary types of acidosis.
Metabolic acidosis
A metabolic acidosis is the most common acid-base disturbance encountered in sick small animals, horses and camelids. A metabolic acidosis is identified by a decreased bicarbonate (HCO3–) and base excess (BE) on a blood gas analysis, and a decreased HCO3– on the chemistry panel.
Metabolic acidosis can be caused by:
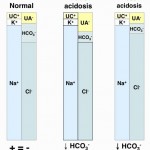
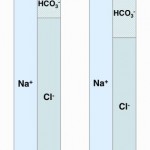
- Consumption of bicarbonate by a non-volatile (non-carbonic) and non-chloride containing acid: This is called a high anion gap or titration acidosis, because the noncarbonic acid increases the anion gap (it is an unmeasured anion, i.e. does not contain chloride as the anion) and the bicarbonate is titrating or buffering the accumulated acid (or the acid is consuming bicarbonate). An alternative term that has been used by some is a “buffer ion” acidosis (Constable 2014). Electroneutrality is maintained because the increase in the unmeasured anion (UA–) that is releasing its proton to cause the acidosis matches the decrease in bicarbonate (HCO3–) that the proton is consuming (see gamblegram to the right). An example of a non-volatile non-chloride containing acid is lactic acid, which has the formula CH3CH(OH)CO2H with the H+ on the end being the acid and the remaining lactate being the “unmeasured” anion accompanying the acid (CH3CH(OH)CO2–). Lactic acid is a strong acid, which means it dissociates readily releasing the free proton (H+), which must be buffered by body buffers, including bicarbonate.
- Loss of bicarbonate or gain of chloride-containing acid: This is called a normal anion gap or hyperchloremic acidosis. An alternative term used with strong ion principles is a “strong ion” acidosis (Constable 2014). When considering bicarbonate loss, think about loss of bicarbonate being accompanied by gain or retention of chloride (with hydrogen, the actual acid part) to maintain electroneutrality (which rules! – see gamblegram). In contrast, direct gain of a chloride-containing non-volatile acid (such as decreased ammoniagenesis in the kidney, leading to retention of NH4Cl) is more intuitive to understand. In these scenarios (loss of bicarbonate or gain of a chloride-containing non-volatile acid), the anion gap does not change, because you have accumulation of a chloride-containing acid, i.e. chloride is the anion (not an “unmeasured anion”, see lactate example above). So think of chloride as the anion of the accumulating acid or in strong ion terms, chloride is a “weak acid”.
These causes/types of acidosis can be differentiated on clinical history (processes responsible for the acidosis), corrected chloride (Cl–corr) and anion gap (AG).
Titration metabolic acidosis
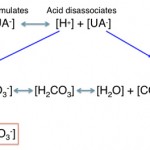
Bicarbonate can be consumed or titrated by a non-volatile non-chloride-containing acid that is produced in the body or is an exogenous toxin, i.e. it is usually pathologic.
- Endogenous acids: Examples of acids produced in the body are lactic acid (from anaerobic metabolism), ketones (diabetes mellitus, ketosis), and acids that are produced from amino acid metabolism and normally excreted by the kidney (phosphates [H2PO4), sulfates [H2SO4]).
- Exogenous acids: Examples of exogenous toxins are salicylate, methanol, ethylene glycol and their metabolites.
The acids (the H+ is released with dissociation of strong acids) are buffered by or consume HCO3– in plasma (UA– goes up, HCO3– goes down), which maintains electroneutrality, therefore the Cl–corr is normal. The anion portion of the non-volatile acids are “unmeasured anions” and their accumulation will increase the AG. Thus, titration or consumption of bicarbonate by a non-volatile non-chloride-containing acid results in a high anion gap metabolic acidosis. With an uncomplicated high anion gap metabolic acidosis, the decrease in HCO3– is roughly equivalent to the increase in AG or unmeasured anions (UA–).
A titration or high anion gap acidosis is a primary acid-base disorder (i.e. it does not occur in compensation to a primary respiratory acid-base disorder). It is the most common acid-base disturbance in most species (except ruminants, such as cattle and sheep, in which a metabolic alkalosis is the most common primary disturbance).
Causes of a primary titration metabolic acidosis include:
- All species: Common acid-base disturbance in most species, including small animals, horses, and camelids.
- L-lactate: From hypovolemia from fluid losses causing decreased tissue perfusion or hypoxia from hypovolemia or severe anemia. This leads to a type A accumulation of lactate from anaerobic metabolism.
- Decreased excretion of normally filtered acids due to kidney dysfunction: This usually occurs with renal azotemia or post-renal azotemia, particularly in acute kidney injury but you can see a high anion gap acidosis with chronic kidney disease (particularly in more advanced stages). In rare cases, may also see with a very severe prerenal azotemia, but the latter likely represents a transient acute kidney injury.
- Small animals:
- Ketoacidosis: Ketones are acids.
- Toxic metabolites (e.g. ethylene glycol, salicylates).
- Cattle:
- Ketoacidosis
- D-lactate acidosis: This occurs particularly in calves, particularly due to fermentation of carbohydrates by bacteria in the colon with intestinal-associated diarrhea or ruminal acidosis from excessive milk intake. Note that D-lactate will not be measured with point-of-care analyzers that provide lactate measurements (these only detect L-lactate). It occurs rarely in adult cattle.
- Camelids: Ketoacidosis and L-lactate.
Bicarbonate loss or gain of a chloride-containing non-volatile metabolic acidosis
This is recognized by a hyperchloremic metabolic acidosis and is due to loss of bicarbonate or a gain of an acid that has chloride as its anion. The anion gap is normal because there is no accumulation of a non-chloride-containing acid (with its unmeasured anion) unless there is another primary disturbance. A hyperchloremic metabolic acidosis can be primary due to bicarbonate loss or gain of a chloride-containing acid or in compensation for a primary respiratory alkalosis.
- Primary hyperchloremic acidosis
- Bicarbonate loss: Bicarbonate is usually lost through the gastrointestinal tract or kidneys.
- Gastrointestinal loss: Causes include vomiting of intestinal contents (pancreatic/intestinal secretions are rich in bicarbonate), secretory diarrhea, and inability to swallow saliva (ruminants, in particular, have lots of bicarbonate in salivary secretions). Intestinal loss from secretory diarrhea is the most common cause of this type of primary acid-base disturbance and is the most frequent cause of a bicarbonate loss acidosis in calves.
- Renal loss: Proximal renal tubular acidosis results in this type of acidosis, because filtered bicarbonate is not being retained or new bicarbonate is not being regenerated. Causes of a proximal renal tubular acidosis (also called Fanconi syndrome) are inherited conditions, e.g. Basenji dogs (Bovee et al 1978), or acquired conditions secondary to toxicity (e.g. jerky toxicosis, lead toxicity, copper toxicity [Langlois et al 2103]) or other causes of proximal renal tubular injury. Proximal renal tubular acidosis is also frequently accompanied by other tubular defects, such as proteinuria in excess for the USG, glucosuria without hyperglycemia and ketonuria. In the kidney, loss of bicarbonate is accompanied by retention of hydrogen with chloride in excess of sodium.
- Since HCO3– is an anion, the body maintains electroneutrality by increasing or retaining Cl–, another anion, with hydrogen. Thus, an acidosis due to HCO3– loss is usually accompanied by a corrected hyperchloremia. The AG will be normal because unmeasured anions are not increased. Therefore, loss of HCO3– usually causes a hyperchloremic normal anion gap metabolic acidosis. With an uncomplicated hyperchloremic metabolic acidosis, the decrease in HCO3– is roughly equivalent to the increase in corrected Cl–. However, it should be noted that some authors attribute the hyperchloremic metabolic acidosis in calves due to loss of sodium in excess of chloride (Constable 2014).
- Gain of a chloride-containing non-volatile acid: Think of chloride as an acid – this is certainly the case when it has hydrogen as its proton (e.g. ammonium chloride or NH4Cl or hydrogen chloride or HCl). The kidney is the main site of retention of chloride with hydrogen, but we can also give acidifying solutions.
- Gain of a chloride-containing acid in the kidney: This occurs with distal renal tubular acidosis (DRTA), when the proton pump (V-H-ATPase) in the distal nephron cannot pump out hydrogen (with chloride usually passively following). Thus, hydrogen with chloride (in excess of sodium) is retained, resulting in a primary hyperchloremic normal anion gap metabolic acidosis. In humans, this occurs with inherited defects in the V-H-ATPase or anion exchanger (AE1) in the distal nephron that causes excretion of hydrogen into the urine. Acquired causes include early chronic kidney disease, immune-mediated disease and certain drugs, e.g. amphotericin (Dhondup and Qian 2017). Proximal renal tubular acidosis, or Fanconi syndrome, can also result in a hyperchloremic metabolic acidosis due to excess bicarbonate loss with retention of chloride by the proximal renal tubules, as mentioned above. However, the metabolic acidosis is usually milder than that with DRTA, because the distal tubules can usually excrete the excess acid and the urine pH can be neutral or even acidic. In contrast, with DRTA, the urine cannot be acidified. Administration of ammonium chloride, as a research tool, also causes a primary hyperchloremic normal anion gap metabolic acidosis. In animals, we rarely see DRTA and a hyperchloremic metabolic acidosis is less common than a titration acidosis in chronic kidney disease. A distal renal tubular acidosis has been reported in dogs with immune-mediated hemolytic anemia (and we have seen cases here as well) (Shearer et al 2009).
- Administration of an acidifying solution: Administration of 0.9% saline can cause a mild acidifying effect (Constable 2014) . It is surprising to think about an isotonic solution such as 0.9% NaCl being potentially acidifying, however this is explained by Constable (2014) by the strong ion difference of the infused solution. An alternative way to consider the acidifying effect of 0.9% saline is that normally in plasma, sodium exceeds chloride (roughly 138-147 mEq/L versus 92-102 mEq/L in cattle). However, by giving equal amounts of sodium and chloride, you may be giving more chloride than is normally present in plasma, creating acidifying situation, using strong ion principles. Note in this scenario, sodium and chloride will still change proportionally in plasma (as you are giving equal amounts). When animals, particularly cattle, are given calcium chloride or diets with negative cation to anion balance (i.e. more anions than cations), this also causes an acidifying effect and cause urinary acidification as a corrective response, via strong ion principles. In the past, a metabolic acidosis was studied by infusing either HCl or NH4Cl (chloride-containing acids) into animals. In these scenarios, chloride will be disproportionally increased compared to sodium.
- Bicarbonate loss: Bicarbonate is usually lost through the gastrointestinal tract or kidneys.
- Compensatory or secondary hyperchloremic metabolic acidosis: The kidney will generate a hyperchloremic normal anion gap metabolic acidosis as compensation for a primary respiratory alkalosis. This is accomplished through:
- Decreased ammoniagenesis in the proximal renal tubules (primarily) so hydrogen is no longer excreted as NH4Cl throughout the kidney. This will result in concomitant loss of bicarbonate.
- Decreased H-ATPase activity in the collecting tubule (primarily), so hydrogen (and chloride) are retained.
- Both of these processes will result in retention of hydrogen and chloride (in excess of sodium) leading to a compensatory hyperchloremic normal anion gap metabolic acidosis.
- A hyperchloremic metabolic acidosis is also the expected attempted corrective response by the kidney to a primary metabolic alkalosis, but this is frequently compromised until we intervene and provide the kidney with what it needs to do its job and fix the primary problem driving the metabolic alkalosis.
The presence of a hyperchloremic normal anion gap metabolic acidosis (low bicarbonate, high Cl–corr) does not mean the acidosis is a primary disorder. A hyperchloremic metabolic acidosis can be primary as indicated above or secondary (or in compensation for) a primary respiratory alkalosis (or the attempted correction for a primary metabolic alkalosis). Whether a hyperchloremic metabolic acidosis is primary or secondary to a respiratory acidosis requires clinical assessment of the patient and knowledge of the underlying disease (e.g. a dog that has small intestinal diarrhea likely has a primary hyperchloremic metabolic acidosis from bicarbonate losses into the intestinal tract). If there is a primary respiratory alkalosis with a compensatory hyperchloremic metabolic acidosis, there will be a clinical disease or condition causing hyperventilation, the blood pH will be more alkaline than acidic (because alkalosis is the primary disturbance) and the pCO2 will be quite low (remember, compensation usually does not return the pH to normal). Kidney function must also be normal for an animal to be able to compensate for a primary respiratory alkalosis.
Causes of a hyperchloremic metabolic acidosis include:
- All species:
- Primary hyperchloremic metabolic acidosis: Secretory diarrhea. Most common metabolic acid-base disturbance in calves (Constable 2014), uncommon in other species. Administration of fluids or diets that have chloride concentrations equal to (0.9% NaCl) or higher than sodium have a mild acidifying effect (e.g. diets with negative dietary cation anion balance, administration of calcium chloride to cattle, as outlined above) (Constable 2014).
- Secondary to a respiratory alkalosis: This is an uncommon cause of a hyperchloremic metabolic acidosis. Requires normal renal function.
- In cases of chronic respiratory alkalosis (>14-30 days) that has been corrected by intervention of some kind, there is a posthypocapnic metabolic acidosis. What starts off as a compensatory response becomes a primary acid-base disturbacne, i.e. a hyperchloremic metabolic acidosis ,with resolution of the primary respiratory alkalosis (hypocapnia). This is because the kidney has not yet had time to eliminate the excess acid that has been retained (but it will self-correct).
- Attempted correction of a primary metabolic alkalosis: The kidney, if functioning properly, will try and correct a primary metabolic alkalosis by excreting NH4Cl or H+ (with Cl) but it is compromised in its ability to do so (see below in metabolic alkalosis).
- Small animals:
- Primary hyperchloremic metabolic acidosis: Proximal or distal renal tubular acidosis, vomiting of intestinal contents because pancreatic secretions are rich in bicarbonate (uncommon).
- Cattle:
- Primary hyperchloremic metabolic acidosis: Loss of bicarbonate in saliva (choke, rabies) in adult cattle. In calves, the most common cause is secretory diarrhea.
- Camelids:
- Primary hyperchloremic metabolic acidosis: Forestomach acidosis (Cebra et al 1996)
Metabolic alkalosis
A metabolic alkalosis is identified by an increased HCO3– and base excess (BE) on a blood gas analysis, and an increased HCO3– and/or decreased Cl–corr on the chemistry panel. A metabolic alkalosis can be a primary disorder or in compensation for a primary respiratory acidosis (or an attempted correction of a primary metabolic acidosis).
Metabolic alkalosis is caused by:
- Loss of a chloride-containing non-volatile acid (chloride-depleted metabolic alkalosis): Loss of these types of acid (e.g. HCl, NH4Cl) causes loss of Cl– without concomitant loss of Na+. Similarly, loss of Cl– in excess of Na+ (chloride acts an “acid” and sodium acts as a “base”) will be alkalinizing. Both will cause (and are recognized by) a decreased Cl–corr. Importantly, these types of metabolic alkalosis are chloride-responsive, i.e. they will be corrected by administration of fluids high in chloride, and are usually associated with volume depletion. This is the most common type of primary metabolic alkalosis in animals but is also the way the kidney compensates for a primary respiratory acidosis (by excreting NH4Cl).
- Renal losses of hydrogen: In rare cases of a primary metabolic alkalosis, renal losses of hydrogen (e.g. stimulation of the V-H+-ATPase in the collecting tubules) can cause acid loss without chloride loss, e.g. primary hyperaldosteronism. The latter type of metabolic alkalosis will not respond to chloride administration and animals usually have normal or increased volume status. Fortunately, it is quite rare.
- Gain of a base or bicarbonate: Gain of bicarbonate (e.g. administration of bicarbonate in fluids) can cause a primary metabolic alkalosis, but this is a far less common cause than loss of a chloride-containing non-volatile acid.
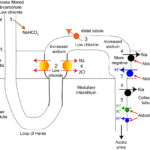
Once metabolic alkalosis begins, other conditions associated with the primary process causing the alkalosis will perpetuate or maintain the alkalosis, specifically hypovolemia, hypochloremia (this is the principal driver of this problem) and hypokalemia. These, particularly the hypochloremia, worsen the alkalosis by causing (in various ways) increased sodium delivery to the distal nephron and increased aldosterone concentrations (via reduced salt sensing in the macula densa). Thus, the kidney, instead of helping correct the metabolic alkalosis actually hurts, by excreting acid (H+) in the collecting tubules (when you really want to retain it to offset the alkalosis) in exchange for sodium resorption. In a nutshell, this is because the kidney becomes sodium avid (wants to retain all filtered sodium as this is the primary determinant of blood volume and sodium retention will help water retention and offset hypovolemia). Both reduced NaCl and hypovolemia induce aldosterone secretion to retain sodium but the retention of sodium comes at the cost of potassium and hydrogen excretion (aldosterone not only stimulates sodium absorption by principal cells in the collecting tubules, it also directly stimulates the V-H+-ATPase in type A intercalated cells in the collecting tubules). In addition, chloride and potassium depletion decrease sodium resorption in the loop of Henle and early distal tubules, thus increasing distal sodium delivery so more sodium is absorbed in the cortical collecting tubules in exchange for hydrogen, particularly when potassium is depleted. So the kidney (primarily the collecting tubules) is largely responsible for persistence of metabolic alkalosis, regardless of cause. We can help the kidney by giving it what it needs to do its job: Volume (replacement fluids), chloride and potassium and fixing the primary problem causing the metabolic alkalosis in the first place. Once the primary problem is no longer driving the pH up and the kidney has these tools, it will be able to excrete the excess bicarbonate and correct the alkalosis (if we have not done so first).
Metabolic alkalosis due to acid loss
Metabolic alkalosis can be secondary to losses of chloride (with or without hydrogen) in a so-called chloride-depleted or chloride-responsive metabolic alkalosis or when there is stimulated excretion of hydrogen with losses in the urine, e.g. primary hyperaldosteronism. In the latter rare disorder, the excess aldosterone directly stimulates the V-H+-ATPase in the collecting tubules causing hydrogen (acid) loss (chloride passively follows but does not control or influence the acid loss) and is thus non-responsive to chloride supplementation.
In an uncomplicated metabolic alkalosis, the increase in HCO3– is usually proportional to the decrease in Cl–corr and the AG is normal. A metabolic alkalosis is a common acid-base abnormality in ruminants with abomasal outflow obstruction (e.g. displaced abomasum) and in small animals with vomiting of gastric contents (most common type of vomiting). The different types of metabolic alkalosis are elaborated on below:
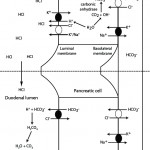
- Primary chloride-responsive (chloride-depleted) metabolic alkalosis: Since H+ is concurrently lost with Cl– in these disorders, patients typically have a low Cl–corr. Excessive loss of Cl– (with respect to Na+) will result in a metabolic alkalosis as HCO3– increases (if you think about maintaining electroneutrality – loss of a negative ion like chloride means a negative ion needs to be retained, which is bicarbonate). It is important to recognize a chloride-responsive metabolic alkalosis (i.e. low corrected chloride = metabolic alkalosis) because the most effective treatment is to provide the chloride back, with sodium- and potassium-containing fluids. Of course, chloride is not the acid, it is the H+ that is with the chloride. HCl is usually lost through the gastrointestinal (primarily vomiting of gastric contents or HCl, see image to the right) or urinary (e.g. diuretics) tracts. These conditions are associated with fluid losses as well and thus you also get volume depletion and sodium avidity, with aldosterone release.
- Gastrointestinal loss: Gastric contents with secretion of HCl are richer in chloride than sodium (e.g. 150 mEq/L chloride versus 110 mEq/L or 120 mEq/L sodium in dogs and cats, respectively). In the gastrointestinal tract, for each milliequivalent of H+ lost in gastric fluid, an equivalent amount of HCO3– will be generated in the intestine and absorbed (see image above). This bicarbonate should be rapidly excreted by the kidneys (filtration and active secretion), however the latter is often impaired in a metabolic alkalosis because of chloride-, volume- and potassium depletion, whereby the collecting tubules act to resorb the excess sodium that is delivered in the distal tubules, resulting in acid excretion (which increases bicarbonate in blood, i.e. excess bicarbonate in blood is linked to increased hydrogen excretion and sodium absorption in the cortical collecting tubule).
- Renal loss: With loop diuretics that inhibit the sodium-potassium-2chloride or NKCC channel (i.e. 2 chlorides for the price of 1 sodium), a chloride-depleted metabolic alkalosis ensues due to increased delivery of sodium to the distal nephron. This also occurs with thiazide diuretics, which block the early distal tubule NaCl carrier (for more information, see renal physiology page relating to sodium absorption). The increased distal sodium delivery causes increased sodium resorption in the collecting tubules, which is directly linked to hydrogen excretion via the Na/H transporter, thus causing bicarbonate retention (equivalent to acid excretion) by the kidneys. Diuretics also cause water loss and hypovolemia (particularly if the animal cannot compensate by drinking) and hypochloremia, both of which may result in aldosterone secretion. Genetic defects in the NKCC transporter (Bartter syndrome) or early distal tubule NaCl carrier (Gitelman syndrome) can cause similar changes as loop or thiazide diuretics, respectively.
- Cutaneous loss: Excess sweating in horses results in loss of KCl and water. There is thus increased distal delivery of sodium (less Na is absorbed in the loop of Henle in the absence of Cl) with subsequent sodium resorption and hydrogen excretion (particularly since potassium is also depleted) in collecting tubules.
- The bottom line is that: With all causes of chloride-depleted metabolic acidosis, instead of the kidney helping fix the problem, the distal nephron is in fact responsible for sustaining or worsening the metabolic alkalosis because of increased sodium delivery and high aldosterone concentrations with subsequent increased sodium absorption linked to hydrogen excretion in the distal tubules. To treat these patients, give chloride (as hypertonic saline), supplemented with potassium.
- Primary metabolic alkalosis that is non-responsive to chloride supplementation: The main example is primary aldosteronism, although genetic defects in the early distal tubule sodium transporter (resulting in increased delivery of sodium to the collecting tubule) will also result in a metabolic alkalosis. These disorders are quite rare in animals.
- Compensatory or secondary metabolic alkalosis: The kidney can compensate for a primary respiratory acidosis by excreting acid while retaining base. This is accomplished through:
- Increased ammoniagenesis in the proximal renal tubules so hydrogen is longer excreted as NH4Cl in the kidney. This will result in retention of bicarbonate and loss of chloride in excess of sodium.
- Increased luminal H-ATPase activity in the collecting tubule (primarily), so hydrogen (and chloride) are excreted.
- Both of these processes will result in excretion of hydrogen and chloride (in excess of sodium) leading to a compensatory metabolic alkalosis.
- A metabolic alkalosis is also the expected attempted corrective response by the kidney to a primary metabolic acidosis, but this is not expected to normalize the pH until we intervene and fix the primary problem driving pH down.
Metabolic alkalosis due to base gain
Administration of NaHCO3 (e.g. treatment of metabolic acidosis) or organic anions (which are metabolized to HCO3–, e.g. citrate in massive blood transfusions), may cause a metabolic alkalosis, particularly under conditions of volume depletion or renal dysfunction, where the kidney acts to retain acid as indicated above. In situations with normal kidney function and chloride concentration, the kidney should rapidly excrete the excess base. A metabolic alkalosis due to gain of base is uncommon.
The presence of a metabolic alkalosis (high bicarbonate, low Cl–corr) does not mean the metabolic alkalosis is a primary disorder. A metabolic alkalosis can be secondary to (or in compensation for) a primary respiratory acidosis. Whether a metabolic alkalosis is primary or secondary to a respiratory acidosis requires clinical assessment of the patient and knowledge of the underlying disease. For instance, if there is a clinical disease causing hypoventilation in a dog and the dog is acidemic (or pH is trending low towards acidemia), with a high pCO2, then there is a primary respiratory acidosis with a secondary or compensating metabolic alkalosis. In contrast, a dog that is vomiting gastric contents likely has a primary metabolic alkalosis (in this case, the pH will be alkaline or trending towards alkaline, unless there is a concurrent primary metabolic acidosis dominating the acid-base picture). Remember compensation does not usually correct pH to normal and over-compensation does not occur. Normal renal function is also required for an animal to be able to compensate for a primary respiratory acidosis.
In summary, causes of metabolic alkalosis are:
- All species
- Primary metabolic alkalosis (chloride-responsive): Vomiting or sequestration of gastric contents (HCl): Common (except in horses, in which it is bad news).
- Secondary in compensation for a primary respiratory acidosis
- In the proximal tubule, increased renal ammoniagenesis with excretion of ammonium chloride (HCl loss with ammonia) promote hydrogen excretion and bicarbonate retention.
- In the collecting tubules of the distal nephron, renal excretion of hydrogen by H+-ATPases (chloride passively follows) and H+/K+ ATPases will promote bicarbonate retention and acid excretion.
- In cases of chronic respiratory acidosis (>30 days) that has been fixed by intervention of some kind, a primary alkalosis can occur, which is called a posthypercapnic metabolic alkalosis. What starts off as a compensatory response, becomes a primary metabolic alkalosis with resolution of the primary respiratory acidosis (hypercapnea). This is because the kidney has not yet had time to eliminate the excess bicarbonate that has been retained. What the kidney needs to do this job is chloride, i.e. a posthypercapnic metabolic alkalosis is chloride-responsive.
- Attempted correction for a primary metabolic acidosis: The kidney tries to correct a primary metabolic acidosis by retaining bicarbonate and excreting acid but as stated above, this is unlikely to normalize the pH unless the primary disturbance is reduced or gone.
- Small animals
- Primary metabolic alkalosis: Gastrointestinal or renal loss of a chloride-containing acid.
- Gastrointestinal loss of HCl: Vomiting of gastric contents; this is most common cause.
- Renal losses of chloride, e.g. loop or thiazide diuretics. Loop diuretics block the Na-K-2Cl carrier in the loop of Henle, so you are losing 2 Cl for the price of 1 Na. Thiazide diuretics block the NaCl cotransporter in the early distal tubule. With both diuretics, there is increased sodium delivery to the collecting tubules, which then resorbs sodium (sodium transporter in principal cells) in exchange for hydrogen or potassium (type A intercalated cells with H+-ATPases in the cortical collecting tubule or H+/K+-ATPases) resulting in acid excretion and retention of bicarbonate (metabolic alkalosis) with concurrent potassium depletion, which helps sustain the alkalosis.
- Other causes are rare, such as Liddle syndrome in humans which is due to increased aldosterone-independent expression of the sodium transporter in the collecting tubules (ENaC) or posthypercapnic metabolic alkalosis.
- Primary metabolic alkalosis: Gastrointestinal or renal loss of a chloride-containing acid.
- Horses
- Primary metabolic alkalosis: This is an uncommon acid-base disturbance due to:
- Gastrointestinal HCl loss: Gastrointestinal issues causing sequestration or reflux of gastric contents (e.g. proximal enteritis, ileus, strangulating small intestinal obstruction, gastric rupture, gastric ulcers). We have seen severe metabolic alkalosis with gastric rupture or reflux in horses. Losses of chloride with specific types of diarrhea (e.g. Potomic horse fever or Neorickettsia risticii) can cause a metabolic alkalosis (the organism interferes with the chloride carrier in the colon). Anecdotally, we have also seen cases of a mild metabolic alkalosis in horses with choke, which may be due to salivary loss. saliva contains equimolar amounts of sodium, chloride and bicarbonate, although this depends on salivary flow rate (Alexander 1966). Equimolar amounts of chloride in salivary actually represents loss of a more acidic fluid because sodium is normally found in serum in higher concentrations than chloride. In one study, ponies with esophageal fistulas that were fed through esophagostomy did develop a metabolic alkalosis with salivary loss, however salivary salt concentration (NaCl) decreased over time, suggesting adaptation of salivary electrolyte secretion. The ponies initially had a metabolic acidosis and due to the decrease in salivary electrolyte secretion, the ensuing metabolic alkalosis was attributed to a renal response (Stick et al 1981).
- Excessive sweating: Loss of KCl.
- Renal loss: Rare (we don’t give them diuretics usually)
- Primary metabolic alkalosis: This is an uncommon acid-base disturbance due to:
- Ruminants
- Primary metabolic alkalosis: This is the most common acid-base disturbance in adult cattle but not calves and is usually due to sequestration of abomasal contents (displaced abomasa, abomasal atony, proximal duodenal obstruction). This occurs in cattle with primary gastrointestinal disease but also other diseases that are associated with or cause secondary abomasal atony, e.g. renal failure. Other causes of chloride loss or metabolic alkalosis are rare or not reported in cattle.
Metabolic summary
The following table provides a summary of the changes in the blood gas (pH, HCO3–, BE) and biochemical panel (HCO3–, AG, Cl–corr) with primary metabolic acid-base disturbances, based on the type of disturbance.
| Disturbance | HCO3– BE |
AG | Cl–corr | Effect on pH |
| Titration metabolic acidosis | ↓ | ↑ | normal | ↓ |
| Bicarbonate loss metabolic acidosis | ↓ | normal | ↑ | ↓ |
| Metabolic alkalosis | ↑ | normal | ↓ | ↑ |
Respiratory acidosis
A respiratory acidosis is identified by an increased pCO2 and low pH (or tendency towards a low pH) on a blood gas analysis. As mentioned previously, the chemistry panel will not provide any information on the respiratory component of acid-base status. A respiratory acidosis is caused by decreased ventilation or gas exchange in the alveoli, which can be secondary to neurologic (affecting the medullary respiratory center), musculoskeletal (affecting the diaphragm and thoracic wall), pulmonary, and cardiac disorders. The most common causes are primary pulmonary disease, ranging from upper airway obstruction to pneumonia, in animals. Note that pneumonia alone unlikely to cause a respiratory acidosis (since pCO2 diffuses so readily across alveolar walls) unless the lung involvement is extensive or there is concurrent respiratory muscle fatigue from a prior hypoxic or pain-induced hyperventilation. Diseases or drugs that inhibit the medullary respiratory center also produce a profound respiratory acidosis, e.g. general anesthesia.
Causes of a respiratory acidosis include:
- All species:
- Primary respiratory acidosis: Respiratory obstruction (uncommon), severe pulmonary disease (usually accompanied by muscle fatigue), inadequate ventilation during anesthesia (iatrogenic).
- Secondary in compensation for a primary metabolic alkalosis: The increase in pH from the alkalosis will be sensed by peripheral chemoreceptors. which will trigger hypoventilation, leading to gain of carbon dioxide (= carbonic acid).
Respiratory alkalosis
A respiratory alkalosis is identified by a decreased pCO2 and high pH (or tendency towards one) on a blood gas analysis. A respiratory alkalosis is caused by hyperventilation. Ventilation is stimulated by central and peripheral (carotid or aortic bodies) chemoreceptors.
- Central chemoreceptors: Respond to pH changes in cerebrospinal fluid (CSF) and hypercapneic hypoxia (characterized by decreased oxygen and increased carbon dioxide ). Changes in CSF parallel changes in blood when there are respiratory disturbances, due to the ready diffusibility of carbon dioxide; pH does not change as readily in CSF with a primary metabolic acidosis, since hydrogen cannot diffuse into the CSF.
- Peripheral chemoreceptors: Respond to hypoxemia (low pO2, e.g. ventilation perfusion mismatch), increased partial pressure of carbon dioxide (pCO2, i.e. correction for a primary respiratory acidosis, when the primary disorder is resolved), and acidemia (low pH or high H+, i.e. the respiratory alkalosis is occurring in compensation for a primary metabolic acidosis). Hypoxemia can be due to respiratory, cardiac or hematological (e.g. anemia, carbon monoxide poisoning) disorders and must be quite low (<50 mmHg) to stimulate hyperventilation, unless there is concurrent acidosis, whereby the body responds to a pO2 < 70-80 mmHg. Hyperventilation can also be stimulated by pain (nociceptors), mechanoreceptors or stretch receptors that are juxtaposed to the alveolar capillaries (e.g. lung disease), marked stress, or anxiety and will then result in a primary respiratory alkalosis.
Causes of respiratory alkalosis include:
- All species:
- Primary respiratory alkalosis: Any cause of hyperventilation (e.g. hypoxemia, pneumonia causing pain, anxiety).
- Secondary in compensation for a primary metabolic acidosis: The body will respond to a decrease in pH sensed by peripheral chemoreceptors by increasing alveolar ventilation and blowing off a volatile acid (carbon dioxide). This is a common cause of a respiratory alkalosis due to the common occurrence of a metabolic acidosis that is primary.
Respiratory summary
The following table provides a summary of the changes in the blood gas (pH, pCO2) with primary respiratory acid-base disturbances, based on the type of disturbance. Note, that a respiratory disturbance cannot be detected from a biochemical panel and a respiratory disturbance does not alter BE.
| Disturbance | pCO2 | Effect on pH |
| Respiratory acidosis | ↑ | ↓ |
| Respiratory alkalosis | ↓ | ↑ |
Mixed disorders
A mixed acid-base disturbance is defined as the presence of more than one primary disturbance. There could be two (not respiratory) or even three primary acid-base disturbances (one respiratory and two different metabolic). Note that it is incorrect to use this term for a single primary disturbance with the appropriate compensatory response. A mixed acid-base disturbance is quite common in animals and should be suspected in these situations:
- The pH is normal but there is an abnormal pCO2 and/or bicarbonate. (Remember that compensation rarely results in a normal pH).
- The change in pH is greater than can be attributed to one disorder alone.
- The pCO2 and HCO3– change in opposite directions (compensatory responses should parallel the primary change).
- The expected compensatory response is:
- Not present and sufficient time has elapsed for it to have occurred.
- Inadequate for what is expected given enough time for it to occur (this is assessed by using compensation formula). Remember it takes longer to the kidneys to fully compensate for respiratory disorder, whereas respiratory compensation is complete in 24 hours.
- Opposite to that which is expected (parallel changes are expected).
- Exceeds that which is expected. For example, in a primary metabolic acidosis, the expected response is a compensatory respiratory alkalosis. If the pCO2 is normal or increased, there is a concurrent primary respiratory acidosis (remember, mild changes may not shift the pH outside the reference interval). The pH would be lower than expected for a primary metabolic acidosis alone, because the combined primary respiratory and primary metabolic acidosis would have an additive effect on lowering the pH.
- The degree of change in acid-base results is not proportional.
- There are easy formulas used to assess for these proportional changes. These formulas depend on whether there is an increased anion gap or not. For all these formulas, the change in test result is compared to the midpoint of the reference interval for the test.
- Change in AG = Measured AG – Normal AG (midpoint of interval)
- Change in bicarbonate = Measured bicarbonate – Normal bicarbonate (midpoint of interval)
- Change in chloride = Corrected chloride – Normal chloride (midpoint of interval)
- Assessment of proportional changes
- In an uncomplicated titration high anion gap metabolic acidosis, the increase in the AG is roughly proportional to the decrease in HCO3– and Cl–corr should be normal.
- In an uncomplicated hyperchloremic metabolic acidosis, the decrease in HCO3– is roughly proportional to the increase in Cl–corr and the AG should be normal.
- In an uncomplicated metabolic alkalosis, the increase in HCO3– is roughly proportional to the decrease in Cl–corr and the AG is usually normal.
- There are easy formulas used to assess for these proportional changes. These formulas depend on whether there is an increased anion gap or not. For all these formulas, the change in test result is compared to the midpoint of the reference interval for the test.
Any deviations from that listed above suggest the likelihood of a mixed-acid disturbance. Remember that changes in serum proteins (mostly albumin) may impact the AG (and should be considered when using these guidelines). Also, do not over-interpret mild changes in electrolytes or other test results; no analyzer or test is perfect!
For example,
- If looking at the anion gap first and you have a high anion gap with a primary titration acidosis and the decrease in bicarbonate is greater than the increase in anion gap, this indicates that there is a mixed disturbance, with something lowering the bicarbonate greater than expected. If the corrected chloride is increased, this would be compatible with a mixed primary high anion gap and primary hyperchloremic (normal anion gap) acidosis, e.g. chronic renal failure, resolving diabetic ketoacidosis, secretory diarrhea with anaerobic metabolism causing a lactic acidosis. Other potential explanations for this scenario are:
- Primary titration acidosis with false decrease in anion gap due to decreased unmeasured anions (very low albumin) or increased unmeasured cations (very high monoclonal immunoglobulins).
- Mixed primary titration acidosis AND primary chronic respiratory alkalosis, with a compensatory metabolic alkalosis. If the pH is high or trending high, body will compensate for the primary respiratory alkalosis by retaining hydrogen and chloride in the kidneys (hyperchloremic acidosis). The latter will decrease the bicarbonate more (and increase the corrected chloride) but have no effect on the anion gap so the bicarbonate will be decreased more than the anion gap is increased.
- If looking at the anion gap first and it is increased and the decrease in bicarbonate is less than the increase in anion gap, this can indicate that there is a mixed disturbance, with something preventing the bicarbonate from being as low as it should be. If the corrected chloride is low, this scenario is compatible with a mixed primary high anion gap acidosis and primary metabolic alkalosis, e.g. gastric dilatation volvulus syndrome in dogs (lactic acidosis with sequestration of HCl-rich fluid), renal failure with vomiting/diuretics, vomiting gastric contents and diabetic ketoacidosis or lactic acidosis. The bicarbonate concentration (and pH) will be dictated by the balance between the two opposing disorders and may be normal. Other potential explanation for these changes are:
- Mixed primary titration metabolic acidosis AND primary respiratory acidosis, e.g. cardiopulmonary arrest, with a compensatory metabolic alkalosis (causing a low corrected chloride). The low pH from two acidoses will drive a compensatory metabolic alkalosis, as long as the kidneys are functionally normally and can excrete acid (with chloride).
- Non-acidotic high anion gap (bicarbonate is normal or high and anion gap is mildly high): Animal has a high anion gap for other reasons, such as increased negative charge on proteins (e.g. severe alkalemia, carbenicillin therapy and dehydration causing increased albumin – these are an uncommon cause of a high anion gap in our experience.
- If looking at the chloride first and there is a decrease in chloride (after correction) that is greater than the increase in bicarbonate, this indicates that there is a mixed disturbance, with something decreasing the bicarbonate. In this example, if there is an increased anion gap, there is a mixed high anion gap acidosis and a primary metabolic alkalosis and the pH and bicarbonate will be dictated by the dominating disturbance (and may be normal).
- It is very difficult to detect a mixed primary normal anion gap hyperchloremic acidosis (which increases chloride and decreases bicarbonate) and a primary metabolic alkalosis (which decreases chloride and increases bicarbonate), because they have opposing effects on chloride and bicarbonate and do not change the anion gap much. This type of mixed disturbance can occur renal failure with vomiting/diuretics, vomiting and diarrhea, and liver disease, but is fortunately, uncommon.
Some examples of mixed acid-base disturbances and the changes that ensue are shown in the table below. Note that not all possible combinations are shown in this table.
| HCO3– | pCO2 | AG | Cl–corr | Disorders | Expected pH |
| ↓ | ↑ | ↑ | ↓ | Primary titration metabolic acidosis (low HCO3– high AG) AND primary respiratory acidosis (high pCO2) AND primary or compensatory metabolic alkalosis (low Cl–corr) – the alkalosis will only be compensatory if the pH is low | N to ↓ (depending on if the alkalosis is primary) |
| N | N | ↑ | ↓ | Primary titration metabolic acidosis (high AG) AND primary metabolic alkalosis (low Cl–corr). If these are balanced and the pH is normal, a compensatory respiratory response will not be triggered. | N (if the dominating disturbance shifts the pH in one direction more than the other, there could be respiratory compensatory changes and changes in pCO2) |
| ↑ | ↓ | N | ↓ | Primary metabolic alkalosis (high HCO3–, low Cl–corr) AND primary respiratory alkalosis (low pCO2) – two alkaloses will increase the pH markedly | ↑↑ |
| ↓↓ | ↓ | ↑ | ↑ | Primary titration AND primary bicarbonate loss metabolic acidosis (very low HCO3– from two acidoses, high AG, high Cl–corr), with a compensatory respiratory alkalosis (low pCO2) because the pH is low | ↓↓ |
The most common mixed acid-base disturbances are:
- Small animals: Titration metabolic acidosis (ketoacidosis, uremic acidosis, lactic acidosis) and metabolic alkalosis (vomiting of gastric contents frequently accompanies these disorders).
- Ruminants: Titration metabolic acidosis (lactic acidosis) and metabolic alkalosis (sequestration of hydrochloric acid due to abomasal atony or displaced abomasa) in adult cattle; titration metabolic acidosis (lactic acidosis) and hyperchloremic bicarbonate loss metabolic acidosis (secretory diarrhea) in calves.
- Horses: Uncommon.
- Camelids: Uncommon.
Related links
- Laboratory detection: Use of laboratory tests to diagnose acid-base disturbances, including more information on bicarbonate measurement and the anion gap calculation.
- Quick test interpretation: A guide to interpreting blood gas results.
- Chloride: Measurement of chloride and interpretation of changes in chloride.
References
- Clinical Physiology of Acid-Base and Electrolyte Disorders by Rose BD and Post DW, 5th edition, 2001. McGraw-Hill, New York, NY.
- Fluid, Electrolyte and Acid-Base Disorders in Small Animal Practice by DiBartola SP, 3rd edition, 2006. Elsevier-Saunders, St Louis, MO.
- Gennari 2011. Pathophysiology of Metabolic Alkalosis: A New Classification Based on the Centrality of Stimulated Collecting Duct Ion Transport. Am J Kidney Dis 58:626.
