Interpretation
Marked neutrophilic inflammation
Chlamydia infection
Explanation
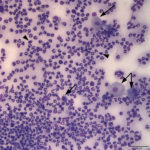
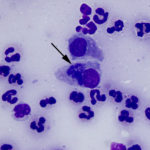
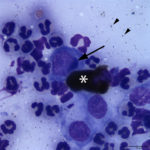
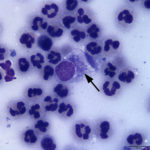
The examined smears were highly cellular consisting of abundant inflammatory cells and moderate numbers of epithelial cells on a proteinaceous background. The inflammatory cells were heavily predominated by non-degenerate neutrophils with few macrophages and small lymphocytes. The epithelial cells were predominantly small polygonal forms with a moderate N:C ratio and fewer more mature superficial forms with a low N:C ratio (both evident in Figure 1A). Moderate numbers of epithelial cells contained small to moderate numbers of fine spindled brown cytoplasmic granules (melanin) (Figure 3A). Low numbers of the more polygonal epithelial cells contained multiple small (<1μm) round deep blue inclusions (presumptive elementary bodies, Figure 4A), as well as more defined, larger perinuclear dark blue inclusions (presumptive reticulate bodies, Figures 2A and 3A). The morphologic appearance of the epithelial inclusions were consistent with Chlamydia.
 |
 |
 |
 |
Discussion
Chlamydiaceae is a family of obligate intracellular bacteria, which have a unique development cycle consisting of two forms.1,2 The elementary body (~0.2-0.6 µm diameter) transiently migrates extracellularly until it infects an epithelial cell by endocytosis.2 The bacteria can survive in the extracellular environment, because of its rigid cell wall.1 Within the cell, the bacteria exists in a cytoplasmic vacuole, where it transforms into the larger, metabolically active replicative form, the reticulate body (0.5-1.5 µm diameter).2 The bacteria within the reticulate body then multiply by binary fission within the cytoplasmic vacuole, becoming a large membrane-bound structure consisting of multiple elementary bodies.2 With cell lysis, the reticulate body ruptures expelling elementary bodies, which can then infect new epithelial cells, repeating the cycle.
Initially, Chlamydiaceae consisted of one genus (Chlamydia) and two species (C. trachomatis and C. psittaci).1 C. trachomatis infected humans, whereas C. psittaci consisted of strains that can infect a wide variety of animals.1 In 1999, with the advent of increased molecular data in the form of 16S rRNA sequences, a division of Chlamydiaceae into two genera (Chlamydia and Chlamydophila) was proposed.1 The number of species also expanded from two to nine. Under the genus Chlamydia included C. trachomatis, C. muridarum, and C. suis, and under the genus Chlamydophila: C. psittaci, C. pecorum, C. pneumoniae, C. abortus, C. caviae, and C. felis.2 Each of these species are fairly host specific affecting 1-3 species.2 The reclassification into two genera was controversial, and as a result in 2009 it was proposed to return all nine species back under one genus (Chlamydia).1 The literature is inconsistent in regards to this recent proposed change and the genus Chlamydophila is still used.
Chlamydia (or Chlamydophila) felis is the species that infects cats, causing upper respiratory signs and mostly ocular signs in the form of conjunctivitis (conjunctival hyperemia, chemosis, blepharospasm, and mucopurulent serous ocular discharge).2 Systemic spread of the infection via uptake of elementary bodies by monocytes rarely occurs.2 Cats aged 2 months to 1 year old are the most likely to be infected, however the presented case is an example of an exception to this rule.2 Kittens younger than 8 weeks old are unlikely to be infected due to passive immunity of maternal antibodies from nursing.2 In experimental infections, clinical signs manifesting as conjunctivitis with or without mild sneezing and serous nasal discharge were seen at 5-10 days post-aerosol exposure with maximal signs of illness at 10-29 days post-exposure.3 Cytologic evidence of Chlamydia inclusions and fever were detected 7-14 days and 11-15 days post-exposure, respectively.3 The two leading differential diagnoses for feline conjunctivitis are feline herpesvirus (FHV-1) and Mycoplasma felis.4,5 Sneezing and nasal discharge without ocular signs are more consistent with FHV-1 than Chlamydia felis infection.2 Co-infections with FHV-1, Mycoplasma, and other agents are also possible, resulting in increased clinical severity of infection.2
Diagnosis can be made on cytologic examination of conjunctival swab material applied to a glass slide. Cytologic assessment has a high sensitivity for identifying Chlamydial inclusions, unlike Mycoplasma organisms and FHV-1 inclusions, which are unreliably or extremely rarely seen, respectively.5 Neutrophilic inflammation is a common finding in cats in the early stages of Chlamydial conjunctivitis.3,4 With chronicity, equal numbers of neutrophils, lymphocytes, and macrophages can be seen.3 Conjunctival nodules of hyperplastic lymphoid tissue can also form around 10-14 days post-exposure.3 Chlamydial inclusions are often perinuclear and appear as dark blue to purple, finely granular structures of ~3-10 µm, which represent clusters of coccoid organisms (0.5-1.5 µm).2,5–7 The chlamydial stage (elementary vs reticulate body) is rarely definitively identified in the figure legends of images in the literature.2,4–7 Some cytology texts4,7 use the term “initial body” to refer to an intermediate stage between the elementary and reticulate body. This term originated in the cytologic description of conjunctival samples from cats experimentally infected with Chlamydia, and was described as a 3-5 µm diameter “amorphous or finely particulate solitary basophilic inclusion”.3 The term initial body is not used in the description of the Chlamydia life cycle2 and, based on its size and cytologic appearance, likely represents the expanding reticulate body during its replicative stage prior to visualizing numerous distinct elementary bodies.
It is important to distinguish Chlamydia inclusions from other infectious agents and non-infectious cytoplasmic material. The main infectious differential diagnosis is Mycoplasma felis, which are small (0.2-0.8 µm) lightly basophilic organisms.4,5 Although a similar size to the elementary bodies of Chlamydia, Mycoplasma organisms are often found along the edge of the cellular membrane, but can be found overlying the cytoplasm making the distinction difficult.4,5 Melanin granules are often seen in conjunctival epithelial cells and are mainly distinguished by their brown color. Topical medications (e.g. Neosporin) have also been associated with visible blue cytoplasmic inclusions in conjunctival epithelial cells, however they appear more irregular and variably sized.8
Chlamydia infection can be confirmed by PCR testing, which focuses on the ompA gene that encodes its major outer membrane protein (MOMP).1 Culture with direct immunofluorescence and a fluorescent-labeled monoclonal antibody specific for the MOMP of C. trachomatis is used to confirm the infection in human patients.9 Although this procedure detects the primary human strain responsible for conjunctivitis, it does not identify other species of Chlamydia. After an initial negative culture, an HIV positive adult male was diagnosed with a chlamydial conjunctivitis that was not due to C. trachomatis, only after the laboratory was notified of the man’s previous contact with clinically ill kittens, prompting the lab to use a Chlamydiaceae specific anti-lipopolysaccharide antibody.9 Molecular analysis of conjunctival samples from the man and his cats confirmed them as indistinguishable isolates of C. felis.9 This is one of few reports of presumptive zoonotic transmission of C. felis.9 Prolonged oral doxycycline treatment is the treatment of choice in cats (and humans) and results in clinical resolution in most cases.2,9
Further information and follow-up
No further diagnostic testing was performed. Based on the cytologic results, a 30 day treatment with a systemic antibiotic (doxycycline) was instituted. The dosing frequency of the ophthalmic antibiotic was increased to every 6 hours. The cat has not presented for a re-check appointment since the writing of this report.
References
- Nunes A, Gomes JP. Evolution, phylogeny, and molecular epidemiology of Chlamydia. Infect Genet Evol [Internet]. Elsevier B.V.; 2014;23:49–64.
- Sykes J, Greene C. Chlamydial infections. In: Greene C, editor. Infectious diseases of dogs and cats. 4th editio. St. Louis: Elsevier; 2012. p. 270–6.
- Hoover E, Kahn D, Langloss J. Experimentally induced feline chlamydial infection (feline pneumonitis). Am J Vet Res. 1978;39(4):541–7.
- Young K. Eyes and Associated Structures. In: Valenciano A, Cowell R, editors. Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat. Fourth. St. Louis: Elsevier; 2014. p. 154–7.
- Hillström A, Tvedten H, Källberg M, Hanås S, Lindhe A, Holst BS. Evaluation of cytologic findings in feline conjunctivitis. Vet Clin Pathol. 2012;41(2):283–90.
- Strik NI, Alleman a R, Wellehan JFX. Conjunctival swab cytology from a guinea pig: it’s elementary! Vet Clin Pathol. 2005;34(2):169–71.
- Campbell T. Exotic Animal Hematology and Cytology. Fourth. Ames: Wiley-Blackwell; 2015. 326-328 p.
- Streeten BW, Streeten EA. “Blue-body” Epithelial Cell Inclusions in Conjunctivitis. Ophthalmology [Internet]. American Academy of Ophthalmology, Inc; 1985;92(4):575–9.
- Hartley JC, Stevenson S, Robinson AJ, Littlewood JD, Carder C, Cartledge J, et al. Conjunctivitis due to Chlamydophila felis (Chlamydia psitacci feline pneumonitis agent) acquired from a cat: Case report with molecular characterization of isolates from the patient and cat. J Infect. 2001;43(1):7–11.
Authored by: Dr. Ashleigh Newman
