Interpretation
Septic suppurative inflammation due to Mycoplasma infection
Explanation
The urinalysis revealed a pyuria (too many WBC/HPF, with <5 WBC/HPF being “normal”), alkalinuria, and trace proteinuria. The presence of increased numbers of WBC in a cystocentesis sample indicates the source of inflammation is located within the bladder, ureters or kidneys. In this case, a cystitis was suspected, based on the thickened bladder wall on ultrasonographic examination. The urine was slightly cloudy due to the epithelial cells and WBC. Although the urine was not very concentrated, the dog was not dehydrated, making it difficult to interpret the urine specific gravity results. This value for urine specific gravity does not indicate the presence of renal disease, unless the dog is concurrently azotemic and no other factors are interfering with urine concentrating ability (e.g. medullary solute washout). Unfortunately, biochemical panel results were not available, but there was no clinical suspicion of renal disease in this patient (renal disease still cannot be ruled out). The polyuria and polydipsia suggest that renal disease may be concurrently present, however there are other causes for these clinical findings and it is possible pollakuria (increased frequency of urination) was mistaken for polyuria, which is increased volume of urination. The degree of proteinuria is borderline (it may be excessive for the urine specific gravity). In this case, it can be attributed to the pyuria (due to exudation of plasma protein into the urine with the inflammation), however underlying renal disease (tubular or glomerular) cannot be ruled out. Note that a positive reaction for protein on a dipstick should not be attributed to protein within the WBC or epithelial cells themselves. The alkaline urine is probably secondary to urea hydrolysis by urease-containing bacteria (e.g. Ureaplasma). Urine pH could also be alkaline if the sample was collected postprandially (post-prandial alkaline tide). The positive reaction for heme can be attributed to the small numbers of erythrocytes in the urine (the dipstick is quite sensitive to red blood cells). The erythrocytes may be secondary to the cystocentesis procedure (numbers are not excessive). A false increase is also possible from microbial peroxidase activity (Question 1).
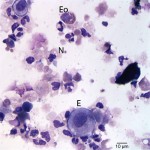
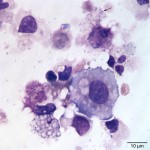
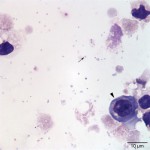
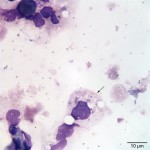
The cytocentrifuged sample of the urine was highly cellular containing inflammatory cells and epithelial cells. Neutrophils were most abundant inflammatory cell (cell “C”, Question 2, Figure 1) and appeared mildly degenerate. The degenerate appearance to the neutrophils could be secondary to the “toxic” environment of urine on cells or the concurrent bacterial infection. Low numbers of eosinophils (cell “B”, Question 2, Figure 1) and vacuolated macrophages (suggesting some chronicity to the infection) were also noted. Low to moderate numbers of individualized round to polyhedral epithelial cells with moderate to high nuclear to cytoplasmic ratios were identified (cell “A”, Question 2, Figure 1). These cells had medium purple cytoplasm and round nuclei with vesiculated chromatin and 1-2 nucleoli. Anisocytosis and anisokaryosis were mild to moderate and rare binucleated forms were seen. This was interpreted as a dysplastic or hyperplastic response, which was attributed to the inflammation. Neutrophils frequently contained non-specific phagocytosed debris, as well as lightly stained coccoid to ring-shaped bacteria (0.5-1 μm), in their cytoplasm (Figures 2-4). The background also contained numerous similarly shaped and sized bacteria (Question 3) and moderate numbers of erythrocytes. The cytologic features of the bacteria are typical of Mycoplasmataceae. These organisms were far too small to be identified on a routine urinalysis (wet preparation of a urine sediment).
 |
 |
 |
 |
Additional testing and follow up
A routine urine culture was negative. However, due to the presence of suspect Mycoplasma or Ureaplasma species on cytologic analysis, a specific culture was requested for these organisms (Question 4). Culture on Mycoplasma-specific media revealed a pure growth of Mycoplasma, which was not speciated further. Unfortunately, no follow up was available for this patient.
Discussion
Mollicutes is a class of wall-less bacteria which includes the family Mycoplasmataceae and the genus of Mycoplasma and Ureaplasma.1 They are smaller than conventional bacteria, measuring approximately 0.3-0.8 um in size. Mycoplasmas are found in various animal (avian, human, mammalian, reptilian), insect and plant hosts. Dogs are first exposed to and colonized by mycoplasmas during birth.1,2 Mycoplasmas are considered to be part of the normal microbial flora of canine mucosal membranes, including the conjunctiva, upper respiratory tract and urogenital tract.3,4 Between 30-50% of male and 23-75% of female dogs have mycoplasmas in the genital tract.1 However, even though they are part of the normal flora, mycoplasmas have been associated with disease, including urinary tract and renal infections (both are thought to be due to ascending infections from the lower genitourinary tract).3 In human beings, it has been suggested that mycoplasmas are responsible for 5% of cases of pyelonephritis.1 Since mycoplasma organisms normally colonize the lower genitourinary tracts, they should not be observed in urine samples collected by cystocentesis. Free catch or voided urine samples, however, could have contaminating bacteria, but due the small size of Mycoplasma, they will not be detected on routine urinalysis and may be overlooked in cytologic specimens (they mimic cellular debris or stain precipitate). It has been suggested that a diagnosis of Mycoplasma infection in the urine should be reserved for those cases in which urine is collected by cystocentesis and when a Mycoplasma is the sole organism isolated on urine culture.3,5 Both of these criteria (cystocentesis, solitary culture) were fulfilled in this current case.
There are 15 known species of Mycoplasma in dogs. Two additional species have been isolated in dogs, but not fully characterized or named. Mycoplasma canis, M. cynos and M. haemocanis are the most important species, being associated with urogenital tract infection/infertility, respiratory disease and hemolytic anemia, respectively. M. canis has been isolated from the prostate, epididymis, chronically inflamed bladder wall and uterus of dogs with urogenital disease and infertility.1,4 The clinical signs associated with urinary mycoplasma infections are not specific for this organism, but can be seen with any cause of bacterial cystitis. These signs include hematuria, polyuria and polydipsia, pollakuria, stranguria, incontinence and abdominal pain.2,3 Mycoplasmas should be considered as a possible cause of urogenital disease in cases of persistent clinical signs despite antibiotic therapy, as seen in this case. Due to their structure (wall-less bacteria), mycoplasmas are inherently resistant to β-lactam antibiotics and prolonged therapy with β-lactams can exacerbate infection.1
In this case, the diagnosis of Mycoplasma or Ureaplasma infection was facilitated by cytologic examination of the urine sediment. As indicated above, caution should be applied if attributing clinical signs to infection in urine samples collected by other methods, due to the possibility of contamination from commensal bacteria in the lower genitourinary tract. On cytologic examination, these microorganisms are smaller than most bacteria and have a variable appearance from coccoid to coccobaccili to signet ring.6 They are frequently observed adherent to epithelial cells,7 which facilitates their identification, but can also be in the background or phagocytosed within inflammatory cells. They can be difficult to differentiate from cellular debris, stain precipitate or non-specific cytoplasmic granularity (particularly in degrading cells). They do not take up gram stain,5 therefore a gram stain would not be helpfu in diagnosis (they will be red on a gram stain but are not truly gram negative). The main differential diagnosis is Brucella canis infection, since these are also small variably shaped coccobacilli, that are gram negative and also associated with infertility. The dysplasia observed in the epithelial cells in this case was attributed to the inflammation. However, underlying neoplasia, that was impeding urine outflow or serving as a nidus for infection, cannot be ruled out. The lack of a bladder mass on ultrasonographic examination does not support an underlying tumor, but does not exclude a carcinoma in situ. Re-evaluation of the bladder wall and epithelial cells in urine was recommended, once the infection had resolved with appropriate antibiotic therapy.
Culture of Mycoplasma species in the laboratory can be difficult, because they require a nutrient-rich environment and are highly sensitive to dryness and heat. A negative culture is frequently yielded on routine bacterial culture on blood agar and special enrichment media should be used. Of the known Mycoplasma species, only M. bovis and M. canis grow in blood agar. Most canine Mycoplasmas (Ureaplasmas and other Mycoplasmas) grow rapidly on commercially available specific mycoplasma media in aerobic conditions at 37°C. Some will also grow under anaerobic conditions. This might explain the lack of growth observed on the routine urine culture performed on this case (suggesting the involved species was not M. canis) and indicates that a specific request for Mycoplasma should be given to a diagnostic laboratory if infection with this organism is suspected (clinically or from cytologic results). The majority of canine mycoplasmas form identical fried-egg like colonies and mixed infections are extremely common, therefore biochemical and serological testing are necessary for the proper identification of Mycoplasma species. Also, polymerase chain reaction for the most commonly isolated organism from dogs are available, however these molecular-based tests are not routinely used.1,2,5 Speciation was not done in this case.
Little is known about the virulence factors and pathogenicity of the canine mycoplasmas.2 Predisposing conditions include urinary neoplasms, urinary calculi, long-term antibiotic therapy and causes of obstruction or urinary stasis (allowing an ascending infection).5 Numbers of bacteria in urine do not necessarily correlate to clinical disease, because more organisms may be present in the chronically thickened bladder wall than in the urine. Indeed, even small numbers of organisms in the urine have been associated with disease and severe clinical signs.2
References
- Chalker VJ. Canine mycoplasmas. 2005. Res Vet Sci. 79:1-8.
- L’Abee-Lund T. et al. Mycoplasma canis and urogenital disease in dogs in Norway. 2003. Vet Rec. 153:231-235.
- Ulgen M. et al. Urinary Tract Infections due to Mycoplasma canis in Dogs. 2006. J Vet Med A Physiol Pathol Clin Med. 53:379-382.
- Doig PA. et al. The Genital Mycoplasma and Ureaplasma Flora of Healthy and Diseased Dogs. 1981. Can J Comp Med. 45: 233-238.
- Craig E. and Victoria J. Nonhemotropic Mycoplasmal, Ureaplasmal, and L-Form Infections, Chapter 32. In: Craig E, ed. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO: Saunders, Elsevier, 2012.
- English K. et al. Transtracheal and Bronchoalveolar washes, Chapter 16. In: Diagnostic Cytology and Hematology of the Dog and Cat. 3rd ed. St. Louis, MO: Saunders Elsevier, 2006.
- Raskin, E. Eyes and Adnexa, Chapter 15. In: Raskin E and Denny J, ed. Canine and Feline Cytology: A Color Atlas and Interpretation Guide. 2nd ed. St. Louis, MO: Saunders, Elsevier, 2010.
