Interpretation
Nasal adenocarcinoma with mixed inflammation and bacterial infection.
Explanation
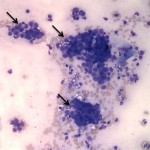
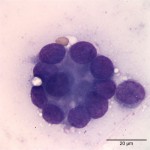
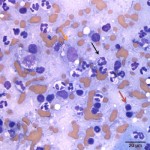
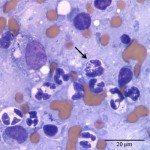
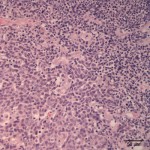
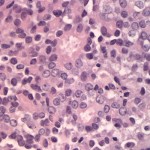
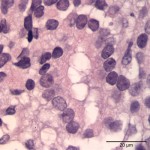
The cytologic smears were highly cellular and composed of neoplastic and inflammatory cell populations (Figure 1). The neoplastic population was characterized by many variably sized cohesive clusters of large cuboidal to columnar cells, with some acinus formation (Figure 2, Question 1). The cells had round to oval nuclei with finely stippled chromatin and one to two indistinct nuclei and a moderate amount of light blue cytoplasm, with indistinct boundaries. They displayed mild to moderate anisocytosis and anisokaryosis. Mitotic figures were not evident. The neoplastic cells were admixed with many mixed inflammatory cells, consisting mostly of mildly degenerate neutrophils with moderate macrophages and lymphocytes, and a few plasma cells (Figure 3, Question 2). Lymphocytes were mostly small cells with low numbers of intermediate or large forms. Many neutrophils and fewer macrophages contained intracytoplasmic rod-shaped bacteria (Figures 3 and 4, Question 2). Some larger rods and coccobacilli were also seen in the background. Macrophages were often vacuolated and contain degrading cellular debris (cytophagia). The cytologic diagnosis of nasal adenocarcinoma and secondary mixed, mostly neutrophilic, inflammation with bacterial infection was made (Question 3).
Additional testing and follow up
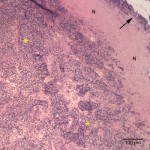
On histopathologic examination of hematoxylin-eosin (H&E)-stained sections of the mass, the normal nasal mucosa, submucosa, and nasal turbinate was markedly expanded and effaced by an unencapsulated, poorly demarcated, and infiltrative neoplasm composed of cuboidal to polygonal cells arranged in sheets, with acinus formation (Figures 5-7). The morphologic features of the neoplastic cells were similar to that observed in the cytologic smears. There was 1 mitotic figure in 10 high power fields (low rate). The nasal mucosa was also multifocally ulcerated and infiltrated by numerous degenerate neutrophils, fewer macrophages, occasional lymphocytes, and plasma cells. The surface of the neoplasm was ulcerated and covered by numerous coccobacillus bacteria (Figure 8). The morphologic diagnosis was a nasal adenocarcinoma with multifocal suppurative inflammation and secondary ulceration with bacterial colonization.
Discussion
The prevalence of feline nasosinal tumors in a clinic population has been reported to be 23 tumors per 10,000 cats1, which is less common than that reported in dogs.1,2 Nasosinal tumors, including squamous cell carcinoma of nasal planum, comprise 8.4% of all feline tumors with the vast majority of the tumors being malignant.1 The mean age of affected animals is approximately 10 years with feline patients as young as 3 years suffering from these tumors.2
Besides squamous cell carcinoma of the nasal planum, nasal carcinoma and lymphoma are the most common nasal tumors in cats.3 Less frequently reported tumor types include sarcomas (fibrosarcoma, osteosarcoma, chondrosarcoma), mast cell tumor, melanoma, plasmacytoma, olfactory neuroblastoma, and benign lesions such as hemangioma, chondroma, and neurofibroma.2 Nasal carcinomas are locally invasive and associated with a low metastatic rate at the time of diagnosis.2
Clinical signs of nasosinal tumors overlap with those of other causes of chronic nasal disease2-4 and include nasal discharge, upper respiratory tract dyspnea, sneezing, epistaxis, facial swelling, ocular discharge, lethargy, regional lymphadenopathy, and weight loss. Although the clinical signs are not specific, animals with dyspnea, unilateral nasal discharge, and/or bloody nasal discharge are more likely to have neoplasia than rhinitis. The median duration of clinical signs before diagnosis is 2 months. Many cats experience temporary alleviation of clinical signs with the use of antibiotics or glucocorticosteroids, as seemed to occur in this case. Differential diagnoses for chronic nasal signs include chronic rhinitis, infectious rhinitis, foreign body, nasal polyp, nasopharyngeal stenosis, and trauma.2
Cytologic examination of traumatic flushes, swabs of the nasal mucosa or imprints of a biopsy can facilitate a definitive diagnosis, however false negative results may occur if only superficial normal, inflamed or ulcerated tissue is sampled (hence a traumatic flush or retrieval of deeper tissue is optimal for cytologic evaluation). Histopathological evaluation of surgical biopsies, without the need for additional immunohistochemical staining, is sufficient to confirm a diagnosis of most cases of nasal carcinoma.5 Surface ulceration with secondary bacterial infection and inflammation as seen in this cat are common concurrent findings.
Malignant nasal neoplasms can be treated with radiation therapy (with or without surgery to remove or debulk the tumor, or both), chemotherapy, or both. The prognosis for untreated malignant nasal neoplasms is poor, with a survival time of only a few months in most cases.2
Further information
After the diagnosis, the cat was treated with antibiotics without surgery or radiation therapy and was still alive 5 months later (at the time of this report).
References
- Willson DW, Dungworth DL. Tumors of the Respiratory Tract. 2002 In: Tumors of Domestic Animals. Meuten D. 4th ed, pp. 365-400. Iowa State Press.
- Withrow SJ. Tumors of the Respiratory System. 2013. In: Withrow and Macewen’s Small Animal Clinical Oncology. Withrow SJ, Vail DM, Page R. 5th ed, pp. 432-462. Elsevier Saunders, St. Louis, MI.
- Mukaratirwa S, van der Linde-Sipman JS, Gruys E: Feline nasal and paranasal sinus tumours: Clinicopathological study, histomorphological description and diagnostic immunohistochemistry of 123 cases. 2001. J Feline Med Surg 3: 235–245.
- Hawkins EC. Disorders of the nasal cavity. 2003 In: Small animal internal medicine, ed. Nelson RW, Couto CG, 3rd ed., pp. 233–235. Mosby, St. Louis, MO.
- Nagata K, Lamb M, Goldschmidt MH, Duda L, Walton RM.2014. The usefulness of immunohistochemistry to differentiate between nasal carcinoma and lymphoma in cats: 140 cases (1986-2000). Vet Comp Oncol 12:52-57.