Interpretation
Mixed cell pleocytosis in both sites, with concurrent suspected hemorrhage in the LS tap.
Explanation
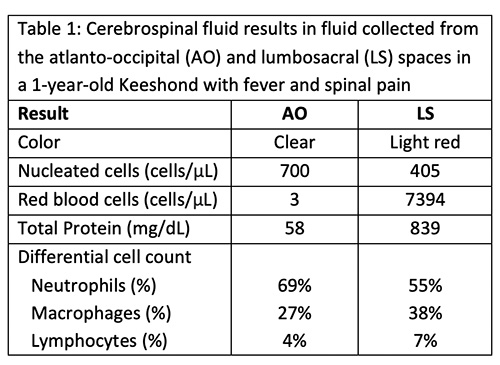
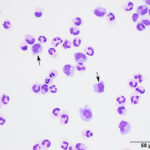
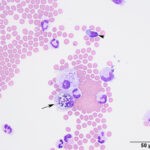
The fluids had high nucleated cellularity (normal, <5 cells/uL) and protein concentration (normal AO, <30 mg/dL; normal LS: <45 mg/dL). Cytospin smears from both sites were cellular and consisted mostly of non-degenerate neutrophils, with moderate macrophages and low numbers of small lymphocytes. Reactive lymphocytes were seen and there were numerous erythrocytes in the smears from the LS tap, commensurate with the higher red blood cell count (Table 1, Figures 1-3, Question 1). In smears from the LS tap, macrophages had distended cytoplasm and displayed phagocytic activity, with purple to dusky gray smooth variably sized round globules and vacuoles in the cytoplasm (Figures 2-3). The duskier grayer globules were considered compatible with hemosiderin, although no erythrophages, indicating acute hemorrhage (<24 hours), were identified. Based on the presenting clinical signs of fever, historical documentation of a systemic inflammatory response, and the cytologic findings in both CSF taps, a diagnosis of steroid-responsive meningitis-arteritis (SRMA) was prioritized (Question 2).
 |
 |
 |
Additional tests
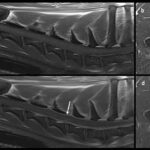
Magnetic resonance imaging (MRI) of the cervical region was performed. The T2-weighted MRI revealed hyperintensities of the spinal cord parenchyma at the level of the third thoracic vertebra and the 5th-6th cervical vertebral disc space, suggesting fluid in the spinal cord. The hyperintensity in these regions was less but still visible on T1-weighted images, supporting inflammation or hemorrhage (Figure 4). The spinal cord meninges were also diffusely thickenedand a focal contrast-enhancing lesion was seen in the cervical muscle. The results, with the clinical signs and signalment and cytologic findings from the CSF taps, led to a final diagnosis of SRMA.
Treatment and follow up
The dog was administered oral gabapentin and pregablin and intravenous fentanyl for pain relief during hospitalization. He was also started on immunosuppressive doses of steroids (1 mg/kg/day prednisone after an initial intravenous dose of dexamethasone), with a gastroprotectorant (omeprazole) and pain medication (pregabalin). One month after discharge, the patient was no longer febrile nor demonstrating clinical signs of cervical or elbow pain. Immunosuppressive therapy was to be gradually tapered every 2 months with the goal of discontinuing treatment if the dog remained free of clinical signs after 9 months of treatment.
Discussion
There are various idiopathic, presumed immune-mediated, diseases of the central nervous system in dogs, including meningoencephalopathies of unknown etiology (MUE), SRMA, eosinophilic meningoencephalitis, non-suppurative meningoencephalitis of Greyhounds, idiopathic tremor syndrome, and hypertrophic pachymeningitis. Meningoencephalopathies of unknown etiology is an umbrella term that encompasses three histopathologic variants of neurologic disease – granulomatous meningoencephalitis, necrotizing meningoencephalitis, and necrotizing leukoencephalitis.1,2 The term MUE is now preferred as the diagnosis of these three diseases is primarily based on clinical, imaging and laboratory findings versus histologic evaluation affected tissues.1 In one retrospective study of 1,140 dogs with inflammatory neurologic disease, immune-mediated conditions comprised 83% of the cases, with MUE being more common than SRMA (57 and 37% of the cases, respectively).1 In another study of 111 cases with a post-mortem diagnosis of meningitis or meningoencephalitis, SRMA only comprised 5% of the cases,3 but the study may be skewed by the higher mortality of the non-SRMA inflammatory disorders. Steroid-responsive meningitis-arteritis is distinguished clinically from MUE by the presence of fever, systemic inflammation, reluctance to move, stiff gait, and pain on manipulation of the neck or thoracolumbar spine. Neurologic symptoms, such as abnormal mentation, ataxia, and decreased proprioception and withdrawal reflexes are seen in less than ¼ of cases.1,4–10 Neurologic deficits are more apparent in dogs which are presented with more chronic disease.5 Other clinical signs include lethargy, anorexia, vomiting, and diarrhea.5,10 Dogs can suffer from multiple clinical episodes.1,8 The dog herein showed typical clinical signs of recent onset disease, with cervical and joint pain, fever, reluctance to move, and no obvious neurologic deficits. With the CSF results showing inflammation and evidence of meningeal involvement on MRI, the clinical signs supported a diagnosis of SRMA.
Dogs with SRMA are mostly of young medium to large breeds, with a median age and weight of 8.5-11 months (range, 2 months – 6.8 years) and 14.1 kg (31 lb), respectively.1,8,10 There is no apparent sex predilection. Commonly affected breeds include dogs of mixed breed, Beagles (the first reported breed with this syndrome, called “beagle pain” syndrome4,7), Boxers, Novia Scotia Duck Tolling Retrievers, and Bernese Mountain Dogs.5,6,8–12 Hound breeds had a 19.1 odds ratio of having SRMA versus MUE in a large retrospective study.1 The reported median duration of clinical signs is 4-6.5 days (range, 1 day – 8.6 months),1,8,10 with most dogs presenting with more established disease (> 4 days).1,6
There is evidence of systemic inflammation in many affected dogs, as seen by a neutrophilic leukocytosis, with or without a left shift, hypoalbuminemia, and increased concentrations of acute phase proteins, such as C-reactive protein (CRP), on clinical pathologic testing.5,8,10 Cerebrospinal fluid analysis in dogs with SRMA usually reveals a mixed cell pleocytosis, with >60% neutrophils and increased total protein concentration,1,5,6,8 as found in our case. Low numbers of dogs can have normal CSF results.9 Immunoglobulin A concentrations can be high in CSF,8 but measurement of IgA in CSF is not routinely performed for diagnosis. The increase in the nucleated cell count is marked in many dogs (>100 cells/uL in 73-75% of cases6,10). In two studies, the median nucleated cell count and total protein concentration varied from 249-945 cells/uL (range, 0-22,000 cells/uL) and 60-65 mg/dL, respectively, and neutrophils comprised >60% of cells in 93% of cases with abnormal CSF results.1,6 In chronic cases (>7 days), the total protein concentration can be normal and the inflammatory population consisting mostly of monocytes with lower nucleated cell counts.5,8 In this case, the CSF taps were done and the diagnosis of SRMA could have been made from either tap. Previous studies comparing CSF from the AO and LS sites in dogs with various neurologic diseases show that the LS is the preferred site for aspiration, yielding a higher percentage of abnormal results regardless of disease localization.13,14 Median nucleated cell counts and total protein concentrations are also higher in the AO versus LS site.14 The AO tap was more frequently abnormal in dogs with cranial or cervical disease than those with thoracolumbar disease.13 In SRMA cases, collecting AO and LS taps appeared prudent as several dogs only had abnormalities in the CSF from one of these sites. The nucleated cell count and total protein concentration can be higher or lower in the AO versus LS CSF samples, with no clear expected pattern in dogs with SRMA.9 In the case herein, a higher total protein concentration, but not nucleated cell count, was seen in the LS versus the AO tap. Putative hemosiderophages were seen in smears only from the LS tap, suggesting intraspinal hemorrhage. Hemorrhage has been documented in the spinal cord in dogs with SRMA, due to a vasculitis, which is found primarily in small to medium cervical spinal arterioles, but can involve vessels in the cortical gray matter and other sites in a few patients. Perivascular inflammatory cell infiltrates can be neutrophilic or mononuclear (macrophages, lymphocytes, plasma cells) with fibrin thrombi and necrosis.3–5,7 Low numbers of dogs with SRMA can have concurrent immune-mediated polyarthritis with suppurative inflammation documented in aspirates of synovial fluid.10 Arteritis causing elbow inflammation was suspected in the case herein.
Advanced imaging with MRI shows evidence of meningeal involvement in >80% of dogs with SRMA with meningeal hyperintensity. Enhancement of nerve roots, synovial linings of vertebral facets, cervical muscles, and spinal cord parenchyma can also be seen on T1- and T2-weighted post-contrast images.6 T1- and T2-weighting differ by the timing of the radio frequency pulses and the time between pulse delivery and signal collection, both of which are longer for T2. The two modes yield different intensities for CSF, fat, muscle, spinal cord, brain and inflammation (https://case.edu/med/neurology/NR/MRI%20Basics.htm). The degree of meningeal hyperintensity correlated to the CSF nucleated cell count in one study of 70 dogs with SRMA.6 In the case herein, MRI revealed hyperintense meninges and parenchyma on T1- and T2-weighted images of the spinal cord and a contrast-enhancing muscle lesion, supporting inflammation or hemorrhage, potentially from the arteritis. However, normal MRI findings in dogs with SRMA have been reported.9
Dogs with SRMA are typically treated with immunosuppressive doses of corticosteroids and rapidly respond to treatment (within 48 hours),8,10,15 as occurred in the dog of this report. Markers of systemic inflammation resolve with treatment as do abnormal CSF results.8 Most dogs go into remission and about 52-75% remain in remission without relapse.8,10,15 Tapering or ceasing prednisone too rapidly can result in relapse. Increased acute phase protein concentrations (CRP, haptoglobin, serum amyloid A) can be seen during relapse and can be potentially used to monitor response to therapy and allow for earlier identification of pending relapses. However, increases in these acute phase proteins are not specific for SRMA and can occur with any cause of inflammation, decreasing specificity of any observed increases. In addition, relapses can occur in the face of normal CRP concentrations.15 Due to patient morbidity associated with high-dose prednisone therapy, other immunomodulatory drugs have been used with corticosteroids, such as azathioprine and cytosine arabinoside, which may allow for lower corticosteroid doses or more rapid tapering.10,16 However, these drugs are also not without their own side effects16 and dogs treated with prednisone alone may have fewer relapses.11
Authors: Abigail DeJohn (class of 2023), and Drs. E. Davies, and T. Stokol
References
- Gonçalves R, De Decker S, Walmsley G, Butterfield S, Maddox TW. Inflammatory Disease Affecting the Central Nervous System in Dogs: A Retrospective Study in England (2010-2019). Front Vet Sci. 2021;8:819945.
- Vitale S, Foss K. Immune-Mediated Central Nervous System Disease-Current Knowledge and Recommendations. Top Companion Anim Med. 2019 Mar;34:22–9.
- Elbert JA, Yau W, Rissi DR. Neuroinflammatory diseases of the central nervous system of dogs: A retrospective study of 207 cases (2008-2019). Can Vet J. 2022 Feb;63(2):178–86.
- Snyder PW, Kazacos EA, Scott-Moncrieff JC, HogenEsch H, Carlton WW, Glickman LT, et al. Pathologic features of naturally occurring juvenile polyarteritis in beagle dogs. Vet Pathol. 1995 Jul;32(4):337–45.
- Tipold A, Jaggy A. Steroid responsive meningitis-arteritis in dogs: Long-term study of 32 cases. Journal of Small Animal Practice. 1994;35(6):311–6.
- Remelli C, Martello A, Valentini A, Contiero B, Bernardini M. Magnetic resonance imaging highlights the meningeal involvement in steroid responsive meningitis-arteritis and suggests the inflammation of the surrounding tissues (70 cases). Front Vet Sci. 2022;9:957278.
- Scott-Moncrieff JC, Snyder PW, Glickman LT, Davis EL, Felsburg PJ. Systemic necrotizing vasculitis in nine young beagles. J Am Vet Med Assoc. 1992 Nov 15;201(10):1553–8.
- Lowrie M, Penderis J, McLaughlin M, Eckersall PD, Anderson TJ. Steroid responsive meningitis-arteritis: a prospective study of potential disease markers, prednisolone treatment, and long-term outcome in 20 dogs (2006-2008). J Vet Intern Med. 2009;23(4):862–70.
- Carletti BE, De Decker S, Rose J, Sanchez-Masian D, Bersan E, Cooper C, et al. Evaluation of concurrent analysis of cerebrospinal fluid samples collected from the cerebellomedullary cistern and lumbar subarachnoid space for the diagnosis of steroid-responsive meningitis arteritis in dogs. J Am Vet Med Assoc. 2019 Nov 1;255(9):1035–8.
- Lau J, Nettifee JA, Early PJ, Mariani CL, Olby NJ, Muñana KR. Clinical characteristics, breed differences, and quality of life in North American dogs with acute steroid-responsive meningitis-arteritis. J Vet Intern Med. 2019 Jul;33(4):1719–27.
- Hilpert E, Tipold A, Meyerhoff N, Schwerdt J, Winkler S, Jurina K, et al. Steroid-responsive meningitis-arteritis in dogs in Germany: Are there epidemiological or clinical factors influencing recurrence rate? Tierarztl Prax Ausg K Kleintiere Heimtiere. 2020 Feb;48(1):5–12.
- Hansson-Hamlin H, Lilliehöök I. Steroid-responsive meningitis-arteritis in Nova Scotia duck tolling retrievers. Vet Rec. 2013 Nov 30;173(21):527.
- Thomson CE, Kornegay JN, Stevens JB. Analysis of cerebrospinal fluid from the cerebellomedullary and lumbar cisterns of dogs with focal neurologic disease: 145 cases (1985-1987). Journal of the American Veterinary Medical Association. 1990 Jun 1;196:1841–4.
- Lampe R, Foss KD, Vitale S, Hague DW, Barger AM. Comparison of cerebellomedullary and lumbar cerebrospinal fluid analysis in dogs with neurological disease. J Vet Intern Med. 2020 Mar;34(2):838–43.
- Biedermann E, Tipold A, Flegel T. Relapses in dogs with steroid-responsive meningitis-arteritis. J Small Anim Pract. 2016 Feb;57(2):91–5.
- Günther C, Steffen F, Alder DS, Beatrice L, Geigy C, Beckmann K. Evaluating the use of cytosine arabinoside for treatment for recurrent canine steroid-responsive meningitis-arteritis. Vet Rec. 2020 Jul;187(1):e7.
