There are several diagnostic techniques that should be used as adjunctive tests to differentiate between different types of acute leukemia (lymphoid and myeloid, i.e. immunophenotyping and cytochemical staining), lymphoma from an acute leukemia (combination of clinical signs and laboratory results), or reactive from neoplastic conditions, e.g. chronic lymphocytic leukemia from a reactive lymphocytosis (immunophenotyping or clonality testing). Results from these tests should always be interpreted in conjunction with results from other clinical pathological testing (hemogram, chemistry, bone marrow or other aspirates) and diagnostic (e.g. imaging) tests. In particular, assessment of morphologic features of the cells in blood or aspirate smears by a clinical pathologist is essential for establishing a diagnosis of hematopoietic neoplasia. It cannot be over-emphasized that the techniques listed below should never be used alone to make a diagnosis of hematopoietic neoplasia.
Immunophenotyping
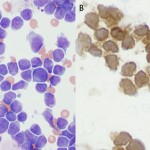
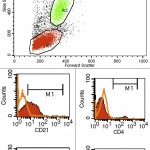
This involves the use of antibodies to detect antigens on the surface or in the cytoplasm or nucleus of cells. Each hematopoietic cell expresses unique markers during differentiation and maturation. Antibodies can be raised against these markers so that they can be used to identify the cell lineage. However, it must be remembered that neoplastic cells can express aberrant markers, i.e. neoplastic lymphoid cells may express myeloid markers, even though their normal counterparts do not. Also, it may be normal (in some species) for some lineage-specific markers to be expressed on more than 1 cell type. For example, CD4 is usually considered a lineage-specific marker of T helper cells but is also expressed by normal neutrophils and activated monocytes or macrophages in the dog. The antibodies can be applied to fixed samples on smears (acetone and formalin are used for cytologic smears or immunocytochemistry or formalin is used for histopathologic sections or immunohistochemistry) or fresh samples in suspension (flow cytometry). In veterinary medicine, we have far more antibodies against antigens of lymphoid lineage cells (T cells, specifically), but relatively few are available for specific myeloid lineage cells (none are available for erythroid progenitors). Antibodies against the following antigens are used for identifying different cell lineages in dogs at Cornell University (there are more available antibodies for dogs than other species). Those marked with an asterix can only be used on samples that are not fixed in formalin, i.e. cells in suspension, cytologic smears or frozen tissue sections.
- Panleukocyte (expressed on all leukocytes to varying degrees): CD45*, CD18 (expressed most strongly on monocytes versus lymphocytes, although this depends on the clone).
- B cell: CD21*, CD22*, CD20, Pax-5, BLA-36, CD79a
- T cell: CD3, CD5*, CD4* (regulatory T cell, dog neutrophil), CD8* (cytotoxic T cell), T cell receptor (TCR)αβ*
- Neutrophil/monocyte: CD11b*, CD11c*, CD80 (Stokol et al 2024)
- Monocyte/lymphocyte: MHCII*, CD11d* (T cell subset, monocyte subset), CD1c* (thymocytes), CD90* (T cell subset)
- Monocyte: CD14*
- Megakaryocyte: CD61*, von Willebrand factor.
- Stem cells: CD34*, CD90*. CD34 is a useful marker for differentiating between lymphoma and acute leukemia, i.e. acute leukemias of myeloid origin are usually positive, whereas lymphomas are usually negative, for CD34 (some B cell lymphomas can express CD34 [Wilkerson et al 2005]).
Cytochemistry
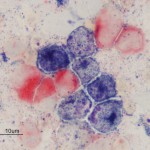
This involves the detection of enzymes within cells by applying substrates for the enzymes to the cells (Jain et al 1991, Stokol et al 2015). If the cells contain the enzyme, they will cleave the substrate forming a specific color that can be detected by light microscopy. Since myeloid cells contain more enzymes than lymphoid cells (except erythroid cells), this is very useful for identifying leukemias of myeloid origin. It is less useful for lymphoid leukemias because they will often be negative for all enzymes. There are also species differences in the enzymes contained within myeloid cells (see table). Common enzyme substrates used in veterinary medicine are:
- Myeloperoxidase (MPx): Neutrophils (mostly), eosinophils (not cat)
- Chloroacetate esterase (CAE): Neutrophils, megakaryocytes (dog), granular lymphocytes can be positive in a focal pattern corresponding to the granules in dogs.
- α-naphthyl butyrate esterase (ANBE): Monocytes, platelets, megakaryocytes. T cells can also stain with this marker, however the staining reaction is more focal than in monocytes where it is more diffuse (there is, however, overlap in staining patterns). The staining pattern in monocytes, but not T cells, can also be inhibited by low concentrations of sodium fluoride.
- Alkaline phosphatase (ALP): Monoblasts (dog only), eosinophils (dog, cat), neutrophils (horse). ALP is generally expressed in most acute myeloid leukemias (exception is an acute megakaryoblastic leukemia as megakaryocytes are negative for ALP). However, T cell leukemias can express ALP, so ALP cannot be used alone to confirm a diagnosis of acute myeloid leukemia versus a T cell neoplasm. B cell neoplasms do not express ALP (Stokol et al 2015).
- Sudan B black (SBB): Neutrophils (mostly) and monocytes (variable, weaker expression than neutrophils). Eosinophil granules stain lightly with this dye. This is a dye that binds specific lipophilic groups in neutrophils and monocytes versus an enzymatic substrate.
- Periodic-acid Schiff (PAS): Stains polysaccharides and mucins (e.g. glycolipids). Mostly positive in neutrophils, with variable positivity in other cell types, including focal positivity in plasma cells.
- Acid phosphatase (ACP): Usually positive in megakaryocytes and platelets, with variable results in other cells.
| Hematopoietic cell | Canine | Feline | Equine |
| Myeloblasts | MPx, CAE, SBB, PAS, ± ACP | MPx, CAE, SBB, PAS, ± ACP | MPx, CAE, SBB, ALP, PAS, ± ACP |
| Differentiating neutrophils | MPx, CAE, SBB, PAS | MPx, CAE, SBB, PAS, ± ACP | MPx, CAE, SBB, ALP, PAS, ± ACP |
| Eosinophil | MPx, ALP (cytoplasm), ± PAS, ACP | ALP, ± PAS, ACP | MPx, SBB, ±ALP, ± PAS, ± ACP |
| Basophil | CAE, ALP, ± PAS, ± ACP | CAE, ALP, ± PAS, ACP | ±CAE, PAS |
| Monoblasts | ALP (strong) | ANBE, ± MPx | ANBE |
| Promonocytes | ALP (light), ± ANBE (diffuse), ± MPx | ANBE, ± MPx | ANBE |
| Monocytes | ± ANBE (diffuse), ± ALP (weak), ± SBB,± MPx, ± PAS, ACP | ANBE, ± SBB, ± MPx, ± PAS, ± ACP | ANBE, ±SBB, ± PAS, ± ACP, ± CAE (Jain, Current Veterinary Therapy) |
| B lymphocyte | ± ALP (normal cells in mantle zone), ± PAS, ± ACP. B cell neoplasms are usually negative for ALP. | ± ACP | ± PAS, ± ACP |
| T lymphocyte | ± ANBE (focal), ± PAS, ± CAE (focal in granular lymphocytes), ± ACP. T cell neoplasms can be variably (light to strong) positive for ALP. | ± ANBE (focal or granular), ± ACP, + ALP (mediastinal lymphoma) | ± ANBE (focal), ± PAS, ± ACP |
| Erythroid progenitor | ± PAS, ± ACP | ± PAS, ± ACP | ± PAS, ± ACP |
| Megakaryocyte | ANBE (diffuse), PAS, ± ACP, ± CAE (chunky granular staining in dogs) | ANBE, ± PAS, ± ACP, ± CAE (slight), ± ALP | ACP |
* Based on published (Stokol et al 2015) and unpublished observations (Julia Blue, Cornell University), a chapter on cytochemistry Schalm’s Veterinary Hematology and Jain’s chapter in Current Veterinary Therapy.
Clonality
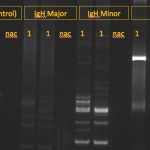
Tests for clonality are based on polymerase chain reaction (PCR) assays for rearrangements in the T cell receptor (for T cell neoplasia) or B cell receptor gene – the heavy chain of IgM (for B cell neoplasia); also called Polymerase chain reaction for Antigen Receptor Rearrangements (PARR). This test is best used for distinguishing between reactive and neoplastic causes of lymphocytosis or confirming a diagnosis of lymphoma versus reactive lymphoid cells in tissue aspirates or histologic sections. It should not be used as a phenotyping tool because clonal rearrangements in B cell receptors can be seen in T cell neoplasms and vice versa (Andrews et al 2016, Keller et al 2016). In addition, some tumors can have clonal rearrangements of both receptors, precluding phenotyping. PCR testing of lymphoid cells from animals with clonal or neoplastic conditions usually have a single band (rearrangement) that will be amplified by PCR. In contrast, animals with reactive lymphoid conditions will have many amplified bands, usually producing a smear on a PCR gel. PCR tests for clonality are excellent for confirming that an expanded population of lymphoid cells is neoplastic, with some notable exceptions. Specific infectious agents and other antigens can cause clonal expansions of lymphoid cells, for instance, Ehrlichia canis can result in a clonal expansion of T cells in dogs, Lyme disease (Borrelia burgdorferi) can cause a clonal expansion of B cells in dogs (Avery and Avery 2007) and Leishmania infection has been associated with both a clonal T or B cell expansion (Melendez-Lazo et al 2019). Note that clonality tests should not be used to distinguish AML from ALL, because many human and animal patients with AML will have clonally re-arranged B or T cell receptors, despite the fact that the neoplastic clone is differentiating along a myeloid (non-lymphoid) lineage (Stokol et al 2017). This could be due to the fact that there is a common monocytic-lymphoid progenitor in marrow. As for any diagnostic test, false positive and negative results do occur.
