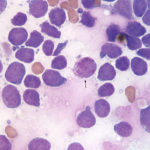
Acute myeloid leukemia (AML) is an aggressive hematopoietic neoplasm, consisting of several different sub-types (as many types of hematopoietic cells there are, there are at least that many types of AML and more). These types differ in phenotypic and genetic characteristics (in humans anyway) and biologic behavior, including progression, response to therapy and prognosis (Swerdlow et al 2008, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edition; also Vardiman et al 2009, Arber et al 2016). Acute myeloid leukemia is the most common type of leukemia in adult humans and in dogs and cats (in our experience). We have inserted images from various animals with AML in this page, but also check out our leukemia gallery under the hematology album, the lymph node album under the cytology album (for AML infiltrates in nodes) and the acute leukemia gallery under the bone marrow album.
Pathogenesis
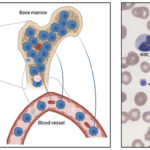
Acute myeloid leukemia involves the clonal expansion of myeloid stem cells, which either minimally, partially or fully differentiate down a particular myeloid lineage (erythroid, megakaryocytic, granulocytic, monocytic). The neoplastic cells have a proliferative advantage and accumulate in bone marrow (where they typically arise), usually at the expense of normal hematopoiesis (either via replacement, an altered microenvironment, or inhibition). Just like other cancer, AML is not a homogenous tumor consisting of one clone, but is rather thought to consist of multiple subclones or leukemia stem cells, which arise from accumulating heterogeneous genetic mutations in an original clone (Welch et al 2012). These stem cells (also called “leukemia-initiating cells”) are quiescent and resistant to chemotherapy and persist in stem cell niches in the marrow. They are thought to be responsible for minimal residual disease and recurrence after remission.
In both humans and animals, AML can arise as a de novo tumor (most common in animals) or secondary to a pre-existing myelodysplastic syndrome (MDS, called myelodysplasia-related AML), treatment for other cancers (called therapy-related AML) not reported in animals) or ionizing radiation (Tolle et al 1982). The most common form of myelodysplasia-related AML is that seen secondary to feline leukemia virus (FeLV) infection in cats (Hisasue et al 2009); this form of AML is very rare in dogs (McManus and Hess 1998). Although dysplasia can be seen concurrently in dogs (Stokol et al 2017), cats (Blue et al 1988) and horses (Barrell et al 2017) with AML, we frequently do not know if this represents a pre-existing myelodysplastic syndrome or just part of the leukemic process. Dysplasia, in itself, is also not diagnostic of an acute leukemia as we have seen dysplasia in hematopoietic cells with lymphoid neoplasms, although it is less common than in AML (dysplasia can also be secondary to chemotherapeutic or other drugs) (Alleman and Harvey 1993, Collicut and Garner 2013).
Acute myeloid leukemia arises due to genetic defects that begin with and then accumulate in the original clone. A vast array of defects have been detected in humans (Welch et al 2012, Papaemmanuil et al 2016); similar studies have not been done with animals and we are still in the dark as to genetic defects in animals with AML. Like other cancers, such as colon cancer, initial genetic mutations are thought to lay the stage for AML development, with additional mutations occurring that result in the tumor. Some of the initial genetic mutations occur in epigenetic modifiers, such as the DNA methyltransferase, DNMT3A, isocitrate dehydrogenase (IDH) and tet methylcytosine dioxygenase 2 [TET2], or chromatin modifiers (e.g. ASXL1). As such it makes sense that altered genetic control will result in additional mutations that precipitate AML. Of note, mutations in epigenetic modifiers, like DNMT3A. are insufficient to cause AML alone and are seen in some older humans (being called clonal hematopoiesis of indeterminate potential or CHIP (Steensma et al 2015)), indicating clonal hematopoiesis and not cancer per se. Other genetic defects, such as gene fusions (e.g. the transcription factors, RUNX1 with RUNX1T1 or the promyelocytic leukemia gene with the retinoic acid receptor alpha [PML-RARA], which results in a leukemia that used to be called promyelocytic leukemia or AML-M3). can result in AML without pre-existing epigenetic mutations. There also may be gene-to-gene interactions, because some combinations of mutations occur in multiple unrelated people (Welch et al 2012, Papaemmanuil et al 2016). Most genetic defects in humans with AML involve mutations or fusions of transcription factors, signaling molecules (e.g. Fms-like tyrosine kinase 3, RAS, KIT), tumor suppressors (e.g. p53), epigenetic modifiers, chromatin modulators (e.g. nucleophosmin), cohesins (proteins that modulate chromatid separation during mitosis, e.g. RAD1, STAG2) and spliceosome proteins (remove introns , e.g. SF3B1, U2AF1). And we should also not forget the microenvironment, which also provides protective niches for leukemic stem cells and may modulate and even trigger leukemogenesis in AML (Shafat et al 2017 review). Data in murine models indicates that leukemic cells cause a state of irreversible senescence in mesenchymal stromal cells in marrow, with subsequent release of cytokines and other factors that promote persistence and proliferation of the tumor cells. Targeting these microenvironmental changes can improve survival in the mouse model of leukemia (Abdul-Aziz et al 2019).
Diagnosis
Since AML usually arises in the marrow and the leukemia effaces the marrow or inhibits normal hematopoiesis, the hallmark laboratory findings are bi- or pancytopenia with or without circulating blasts. In both humans (Ganzel et al 2016) and animals (Barrell et al 2017, Stokol et al 2017, Cooper et al 2018), neoplastic cells can also infiltrate extramedullary sites, often in a vascular-oriented distribution, including the liver, spleen, and lymph nodes and infrequently other organs, including the skin, gastrointestinal system, and brain. In some patients, the tumor can efface infiltrated organs such as lymph nodes or form extramedullary masses, called myeloid sarcoma (aka chloroma, granulocytic sarcoma). Myeloid sarcoma is rare in animals, but we have seen it most commonly in dogs with AML, where it manifests as a variably sized (often large) mediastinal mass, that mimics thymic lymphoma (Epperly et al 2018). Some of the latter dogs can even have normal hemograms and aspirates of lymph nodes or internal organs with AML infiltrates can be readily misdiagnosed as lymphoma. A diagnosis of acute leukemia (of undetermined lineage) can be readily made when there are large numbers of circulating blasts and concurrent cytopenias of normal hematopoietic cells. Then the leukemia must be classified further into AML, acute lymphoblastic leukemia (ALL) or leukemic phase of lymphoma with more testing, including immunophenotyping and/or cytochemical staining. In the absence of circulating blasts, the diagnosis of AML requires finding ≥20% myeloid blasts in a bone marrow aspirate, with myeloid lineage determined by the aforementioned tests (not ever clonality tests for lymphoid gene rearrangements or PARR). We also recommend that as many markers as there are available be thrown on cases of acute leukemia. Cross-lineage expression of lymphoid antigens (e.g. CD3, CD5, CD22) can and does occur in AML (Stokol et al 2017) and we have seen expression of antigens that are normally restricted to neutrophils and monocytes (CD11c on granular lymphocyte tumors and rarely CD11b) on lymphoid tumors (lymphoma, ALL, or chronic lymphocytic leukemia). For this reason, we use the balance of evidence to distinguish between AML and an infiltrative lymphoma (in the marrow) or ALL, i.e. do the tumor cells express more myeloid than lymphoid morphologic features, antigens and cytochemical stains? At Cornell University, we will use morphologic features with flow cytometric-based immunophenotyping (readily done on blood, bone marrow and tissue aspirates) as the first line diagnostic tests to distinguish AML from lymphoid neoplasms. If that is insufficient to sway us one way or another (e.g. leukemias in dogs that are only positive for the stem cell marker CD34+and negative for differentiation markers and MHCII-), then we resort to cytochemistry in an attempt to get a final answer. Why do we care? Because we treat them differently – AML with doxorubicin-cytosine protocols and lymphoid tumors (ALL or lymphoma) with CHOP. Unfortunately, we lack the genetic markers that characterize so many types of AML in human patients (Arber et al 2016), leaving us with morphologic features, immunophenotyping and cytochemical staining to confirm myeloid lineage and provide a diagnosis of AML.
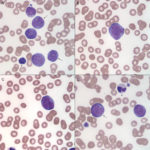
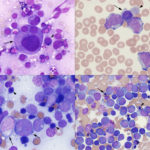
- Morphologic features: A diagnosis of AML requires that there are 20% or more myeloid blasts in marrow (or in the absence of marrow, in venous blood). Erythroid leukemia is a special case, because a cut off of 30% or more erythroid blasts is accepted when erythroid precursors dominate in marrow (Arber et al 2016). On rare occasions, we have made a presumptive diagnosis of AML when there are infiltrates of blasts in peripheral tissues (e.g. lymph nodes) with other supportive findings of AML (e.g. marked dysplasia in multiple hematopoietic lineages). However, it should be noted that tissue infiltrates of AML are often misdiagnosed as lymphoid neoplasia on cytologic and histologic specimens (in our experience, AMLs are often diagnosed as non-B and non-T cell lymphomas, after they are negative for classic T and B cell markers on histologic sections).
The basic premise is to recognize a blast. What is a blast? Obviously, this is going to vary between clinical pathologists (subjective), even with defined criteria in humans (Mufti et al 2008) and described features in animals (Jain et al 1991, Stokol et al 2017, Cooper et al 2018). Here, we define blasts as immature cells (of as yet undetermined lineage), meaning that they have fine chromatin (euchromatin) and may or may not have nucleoli. They can vary in size from small to large (and some are definitely mis-identified as small lymphocytes) and generally have round nuclei, but nuclei can be indented, irregular or indented (particularly in monoblasts). Nucleoli do not make or break a blast, particularly as nucleoli can be inapparent in modified Wright’s stains, which are in standard use for cytologic smears in animals. Blasts can be agranular or contain pink to red (usually small and not in vacuoles, like granular lymphocytes) to purple granules (latter can be chunky and often overlie the nucleus, unlike mast cell granules). As for human criteria (Swerdlow et al 2008), promonocytes are included in the blast count for myelomonocytic, monocytic or monoblastic variants of AML. Promonocytes have more variable nuclear shapes than blasts, being more deeply lobulated or irregular and on the way to becoming monocytes (monocytoid), but with higher nuclear to cytoplasmic ratios and finer chromatin than normal monocytes. Proerythroblasts (aka rubriblasts) in acute erythroid leukemia have round nuclei, fine chromatin and deep blue slightly grainy cytoplasm, that may contain a perinuclear clear zone. Megakaryoblasts can have ruffled borders and cytoplasmic blebs with pinker smoother cytoplasm than typical myeloblasts. To lean towards a diagnosis of AML over ALL, we look for morphologic features of myeloid differentiation (listed in the table below). However, morphologic features alone are unreliable in making a definitive diagnosis of AML (unless all we have is morphology, when immuno- and cytochemical stains are negative, e.g. erythroid and possibly megakaryoblastic leukemia) and should not be relied upon. - Immunophenotyping: This is usually done by flow cytometry but can be done on cytologic or histologic tissues. Staining of cytologic smears is time-consuming and labor-intensive and beset with problems of ruptured cells and false positive staining reactions (e.g. in macrophages). Staining of histologic smears is limited by the few markers which work in formalin-fixed tissue (e.g. CD3 for T cells; Pax-5, CD20, BLA-36, and CD79a for B cells, CD18 for all leukocytes in dogs, but stronger on myeloid than lymphoid cells; Iba-1 and MAC387 for macrophages; CD172a for neutrophils and monocytes in horses; myeloperoxidase for neutrophils and eosinophils (not in cats); von Willebrand factor or CD61 for megakaryocytes). As stated above, throw as many markers as you can at an acute leukemia and use the preponderance of evidence, recognizing that cross-lineage expression of antigens and aberrant expression of markers occurs in AML (Stokol et al 2015, Barrell et al 2017, Stokol et al 2017). Flow cytometric staining patterns should never be interpreted alone but always in combination with hematologic and bone marrow results and morphologic features of the cells in question. It also should be noted that published immunophenotyping characteristics of specific hematopoietic tumors are entirely dependent on the technique used by the authors, particularly on the clones used. The use of different clones or techniques for analysis may not yield in repeatable results across institutions or studies. We have associated certain patterns of antigen expression with AML in dogs. Far less is known in cats (way fewer antibodies available) and horses (way fewer cases with AML). For positive staining reactions, we use a cut-off of ≥20% (beyond the isotype control) except for CD34, in which we use ≥5% (not expressed in normal canine blood).
- Cytochemistry: Although this appears to be no longer used for diagnosis of leukemia in animals (having been supplanted by immunophenotyping), we still perform this test sequentially when flow cytometric results are inconclusive or as a second line test (after routine clinical pathologic testing and assessment of morphology) if flow cytometry has not or cannot be performed (see table below, Stokol et al 2015 and 2017). Cytochemical staining is also used to help diagnose AML in humans (Swerdlow et al 2008, Vardiman et al 2009). Cytochemical stains have specific staining patterns in normal cells of various species (see classification tools). However, a leukemia can and does do what it wants and aberrant staining can occur and should not be unexpected. So again, use the majority of evidence to help support a diagnosis of AML. A cut-off of 3% positive cells is used (Swerdlow et al 2008, Stokol et al 2015 and 2017).
- Clonality testing: Since clonal rearrangements in B and T cell receptors does occur in AML in dogs (Stokol et al 2017), clonality testing should not be used to distinguish between AML and lymphoid tumors. Others have recommended that clonality testing also not be used to phenotype lymphoid neoplasms either (Keller et al 2016).
| Morphologic features |
|
| Immunophenotyping results (antibodies used at Cornell with flow cytometry) |
|
| Cytochemical stains (used at Cornell University) |
|
Classification
We base the classification on AML on the 2008 updated and 2016 revised WHO criteria (Swerdlow et al 2008, Vardiman et al 2009, Arber et al 2016). The WHO scheme has 6 different categories: 1) AML with recurrent genetic abnormalities, 2) AML with myelodysplasia-related changes, 3) Therapy-associated AML, 4) AML-NOS, 5) Myeloid sarcoma and 6) Myeloid proliferations related to Down’s syndrome. Since we currently do not know what genetic abnormalities are present in animals and some of these disease (Down’s syndrome) do not occur or are rare (therapy-associated), most of the AML in animals falls into the AML-NOS category (see Table below). This category is based on the original classification scheme created by a French-American-British working group (Bennett et al 1976) and was first applied to animals by the Animal Leukemia Study Group (Jain et al 1991) and is still in use today (with some modifications, such as we use a blast cut-off of ≥20% to differentiate AML from myelodysplastic syndrome versus the original ≥30% cut-off).
| AML subtype | Features | Comments |
| AML with minimal differentiation (M0) | No evidence of myeloid differentiation morphologically or with cytochemical stains. Blasts are agranular. Myeloid origin confirmed by immunophenotyping (expression of stem cell markers and early myeloid antigens e.g. CD13, CD33 [not available in animals]) or ultrastructure. Rarely express lymphoid antigens in humans. | Difficult to confirm in animals (we lack markers) |
| AML without maturation (M1) | Blasts (>3%) are positive on cytochemical staining (e.g. MPx, SBB) or contain Auer rods (not seen in animals); blasts can be granular. Express early myeloid-associated antigens but no or minimal differentiating antigens on immunophenotyping. Can express lymphoid antigens (e.g. CD7, CD2, CD4). | Reported in animals (e.g. Jain et al 1991) |
| AML-M2 with maturation (M2) | Evidence of neutrophilic differentiation (progranulocytes, myelocytes and mature cells comprise ≥10% of marrow cells) with differentiating monocytes being <20% of marrow cells and blasts being positive for myeloid markers (CD4, CD11b, CD11c and not CD14 in animals versus CD15 or CD16 in humans) on cytochemical staining or immunophenotyping. Can express lymphoid antigens in humans. | Reported in animals (e.g. Jain et al 1991) |
| Acute basophilic leukemia (M2B) | Blasts contain coarse basophilic granules (which can resemble mast cell or basophil granules ultrastructurally in humans) with few mature basophils. Blasts are positive for toluidine blue and negative for other cytochemical stains (MPx, SBB, CAE, ALP, ANBE) in humans. Blasts express early myeloid and differentiating myeloid antigens (e.g. CD11b) and may express stem cell markers, but sually lack CD25 (mast cells) and lymphoid antigens in humans. | Reported in animals, with eosinophilic variants (e.g. Cooper et al 2018) |
| Acute myelomonocytic leukemia (M4) | Consists of differentiating neutrophils (≥20% of marrow cells) and monocytes (≥20% of marrow cells) with promonocytes included as blasts. Blasts may express both granulocyte (CAE, MPx) and monoblast (ALP in dogs) cytochemical stains, with some cases having dual positive cells. Canine cells express differentiating granulocyte and monocyte antigens (CD11b, CD11c, CD14, CD4, and >10% CD14/CD4 double positive cells. Small blasts may express CD34 but are negative for MHCII in dogs but many are negative for CD34. Monocytes are aberrantly negative for MHCII. Equine cells express CD14/CD163 and CD172a. Lymphoid antigen expression is rare in humans, but can be seen in dogs. Often quite marked dysplasia in neutrophils (and monocytes or other lineages) in dogs. Can infiltrate extramedullary site and cause myeloid sarcomas in dogs. | A common type in dogs (Villiers et al 2006, Stokol et al 2015 and 2017) |
| Acute monoblastic (M5a) and monocyte (M5b) leukemia | Consists of differentiating monocytes (>20% of marrow cells) with most cells being monoblasts/promonocytes in acute monoblastic leukemia and promonocytes/monocytes in acute monocytic leukemia. Can have a neutrophilic component but is <20%. In dogs, monoblasts are strongly LAP positive, ± ANBE positive and often only express stem cell markers (CD34, CD90) and lack MHCII expression. They do not demonstrate monocyte differentiation markers (CD11b, CD11c, CD14, MHCII) and can sometimes express CD5 (usually a subset or weak). Monocyte variants usually (but not always) express differentiation antigens (CD14). They are weakly positive to negative for ALP and positive (diffuse pattern of staining) for ANBE. They usually express CD34 but not MHCII. In humans and dogs, these frequently infiltrate extramedullary sites and can form myeloid sarcomas in dogs. Hemophagocytic in humans. | Common in dogs (Villiers et al 2006, Stokol et al 2015 and 2017) |
| Acute erythroid leukemia, pure erythroid type (M6) | Consists of >50% nucleated erythrocyte precursors, with >30% proerythroblasts (rubriblasts) and <20% non-erythroid myeloblasts (of total marrow cells (those with ≥20% myeloblasts are put in other AML categories in the latest WHO criteria). These, unfortunately, are negative on all cytochemical stains and no antibodies against specific erythroid precursor markers are currently available, therefore the diagnosis is based on morphologic features (cells with very round nuclei and deep blue cytoplasm) | Most common type in cats (Jain et al 1991). Rare in dogs (Tomiyasu et al 2011) |
| Acute megakaryoblastic leukemia (M7) | Consists of ≥50% cells of megakaryocytic lineage. Blasts may have pseudopodia or cytoplasmic blebs and usually see dysplasia in megakaryocytes, such as micromegakaryocytes (and other lineages). The marrow may be fibrotic in humans. Blasts may express ANBE and may be positive for acid phosphatase but are negative for other myeloid stains (SBB, MPx, CAE). Blasts may express CD61 and often lack CD34 in dogs. On immunohistochemical or immunocytochemical staining, they can also be positive for von Willebrand factor, CD41 or CD61. | Rare in dogs, cats (Colbatsky and Hermanns 1993, Comazzi et al 2010). Not reported in horses |
| Acute panmyelosis with myelofibrosis | Marrow is fibrotic (with reticulin fibers and not necessarily collagen) but with hypercellular areas containing increased precursors of all lineages. Most difficult variant to identify blast percentage (most are 20-25%) in humans. Difficult to distinguish from the chronic myeloproliferative disorder, idiopathic myelofibrosis. | Not reported in animals |
Specific species
Dogs
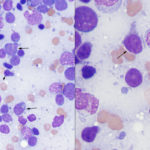
Acute myeloid leukemia is most frequently recognized in middle aged dogs (Vernau and Moore 1999, Villiers et al 2006, Adam et al 2009, Tasca et al 2009, Novacco et al 2016, Davis et al 2018) versus other species and, in our experience, are usually myelomonocytic or monoblastic/monocytic variants. Presenting clinical signs are not specific (anorexia or inappetence, lethargy and vomiting) and there is no breed or sex predilection. The disease can be diagnosed in very young dogs (under a year of age) so age is not a barrier for this leukemia. The diagnosis should be suspected if abnormal cells (“big blue cells“) are seen in peripheral blood with concurrent bi- or pancytopenia (varying combinations of anemia, usually but not always non-regenerative, neutropenia and thrombocytopenia). Most dogs have high numbers of blasts, but those with low numbers of blasts can be mistaken for lymphoma. Rare dogs can even have normal hemograms (Adam et al 2009, Stokol et al 2017). In addition, some dogs can have solitary or multiple peripheral lymphadenopathy, abdominal organomegaly (spleen, liver) and mediastinal masses or present with body cavity effusions (see February 2018 Case of the Month), clinical findings commonly associated with lymphoma. Indeed, we have had several cases referred to us with a presumptive diagnosis of lymphoma that end up having AML. We and others have misdiagnosed AML as lymphoma on tissue aspirates or in body cavity effusions, particularly when the organs are effaced or heavily infiltrated and myeloid differentiation is lacking. Clues as to an AML in a tissue aspirate include blasts with pleomorphic nuclei and of variable sizes (also can be seen with some variants of lymphoma), blasts with variable cytoplasmic colors (lighter and darker blue, unusual for lymphoma), a residual population of normal lymphoid cells (in lymphoid organs) indicating an infiltrative population, and dysplasia in differentiated hematopoietic lineages (see image above).
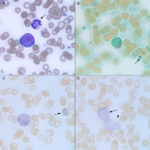
As stated above, a bone marrow aspirate is required for diagnosis of AML on the basis of 20% or more myeloblasts (except erythroid variants, which are rare), however venous blood is used as surrogate for bone marrow in some dogs when there is a marked leukocytosis of blasts. By the time dogs present with AML, the marrow is effaced (>60-80% tumor cells), readily leading to a diagnosis of acute leukemia (myeloid, lymphoid, mixed lineage or undifferentiated), with lineage being ascertained, when possible, by additional immunophenotyping or cytochemical staining. In those cases with fewer blasts, the main distinction is an infiltrative lymphoma, which is readily done with immunophenotyping and assessment of the animal for the primary lymphoid tumor in extramedullary sites. At our institution, discrimination between AML and ALL is desired in a dog with acute leukemia because anthracycline/cytosine arabinoside- and CHOP-based protocols are the treatment of choice for AML and lymphoid neoplasms, respectively. Unfortunately, the prognosis is very poor, with most dogs being euthanized shortly after diagnosis or within 1-4 months after initiation of chemotherapy.(Villiers et al 2006, Novacco et al 2006, Davis et al 2018)
Cats
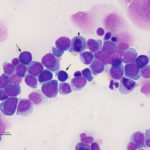
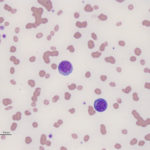
Acute myeloid leukemia is less common in cats versus dogs (Jain et al 1991) and is frequently secondary to infection with FeLV (Hisasue et al 2009), particularly in those cats with concurrent myelodysplasia. The AML can be diagnosed simultaneously with MDS or MDS can precede the AML by months to years (Madewell et al 1979, Toth et al 1986, Hisasue et al 2009). Due to the association with FeLV, younger cats are affected by AML, but like dogs, they have similar non-specific presenting signs and there is no sex or breed associations. Like dogs, cats can have high numbers of circulating blasts and cytopenias (one or multiple lineages) is typical. Since many cats with AML have erythroid leukemia or pre-existing or concurrent myelodysplastic syndrome (MDS), normoblastosis (increased nucleated red blood cells) is a common finding and proerythroblasts and myeloblasts can be seen in circulation or marrow. Due to the association with FeLV and the myelodysplasia, affected cats usually have macrocytic erythrocytes (high MCV) without a reticulocytosis and macrocytic RBCs usually lack detectable RNA on methylene-blue stained smears (i.e. are not punctate reticulocytes). As for any species, infiltrates can occur in other organs (liver, spleen) but lymphadenopathy is seen less frequently than in dogs. On bone marrow aspirate, other than the mandatory ≥20% myeloblasts (in non-erythroid variants, see above table) or ≥30% proerythroblasts (called rubriblasts in veterinary medicine) in cases with ≥50% erythroid progenitors, we often see myelofibrosis, which is not a common finding in marrow of dogs or horses with AML; myelofibrosis is also a feature of MDS in cats (Blue 1988). We also find Prussian blue-stained smears for iron useful, because iron stores can be apparent in macrophages and in nucleated erythroid precursors (sideroblasts) or mature RBCs (siderocytes) in both AML and MDS in cats (Blue 1988, Blue et al 1988). Iron inclusions in RBCs is rarely seen in immune-mediated or other marrow disorders in cats (Blue 1988). In addition, features of dysplasia are common in hematopoietic cells in blood and marrow in cats with AML, due to the reason outlined above, whereas in dogs, it is more seen in subtypes of AML (acute myelomonocytic and megakaryoblastic in particular). Like other species, the prognosis is poor with most cats being dead within 2 months of diagnosis (Blue et al 1988, Jain et al 1993).
Horses
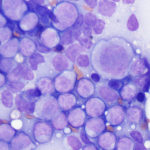
As for dogs and cats, horses with AML range from young to older, although in two cases, most horses were between 2-15 years of age (Barrell et al 2017, Cooper et al 2018). The disorder is far more uncommon than in dogs; we have only seen 6-7 cases over 25 years. Typical hematologic findings in horses are bi- or pancytopenia with low numbers of circulating blasts, which contrasts with many dogs, in which blasts dominate in blood, and cats, in which blasts or dysplastic changes are frequently evident. Blasts in most horses, in our experience, lack cytologic features of myeloid differentiation, with exceptions (of course, Barrell et al 2017). Some horses can have concurrent myelodysplasia and we have seen isolated cases of MDS in horses (unpublished data); it is possible that MDS was pre-existing in affected animals. Some of these horses can have macrocytic RBCs, like cats. Since circulating nRBCs are an exceedingly uncommon finding in horses, their presence should be a red flag in a horse with cytopenias, supporting a bone marrow problem. Clinical signs in horses are non-specific and include edema. Lymphadenopathy of single or multiple peripheral lymph nodes can be seen and lymph nodes can be extensively infiltrated on aspiration (see image). In bone marrow aspirates, horses can have variable numbers of blasts and the marrow may not be effaced (>80% blasts). Some horses have concurrent evidence of inflammation (left shift and/or toxic change in neutrophils, polyclonal gammopathy on electrophoresis, increased concentrations of acute phase proteins like serum amyloid A) and infections (Barrell et al 2017, Cooper et al 2018). Nodular masses can be seen, mimicking lymphoma (Cooper et al 2018) and infiltrates can occur in multiple sites, including the brain and gastrointestinal tract (Barrell et al 2017, Cooper et al 2018). Again the prognosis is poor, with most horses being euthanized or dying from the disease within a few days to a month after diagnosis (Barrell et al 2017, Cooper et al 2018).
Other species
Acute myeloid leukemia is rarely reported in ruminants (Woods et al 1993, Laabs et al 2015) and exotic species, such as bearded dragons (Hruban et al 1992, Tocidlowski et al 2001). We have also seen cases (as yet unpublished) in bearded dragons, hedgehogs and porcupines, but we sorely lack cross-reactive antibodies for lymphoid or myeloid antigens to confirm a diagnosis of AML or exclude lymphoid neoplasia and cytochemical reactions in normal animals from such species are not completely known, hindering diagnosis (or relying on morphologic features in cytologic or electron microscopic samples).