Interpretation
Marked suppurative inflammation (exudative effusion) with barium crystals
Explanation
Direct smears, prepared from the submitted peritoneal fluid, were moderately to highly cellular, consisting of inflammatory cells in a small amount of blood. A differential leukocyte count was 96% non-degenerate neutrophils and 4% macrophages. There were many variably sized, irregularly shaped, pale yellow to colorless, refractile material (barium, presumptive) in the background, as well as phagocytized within neutrophils and macrophages (Question 2) (Figure 1a-2a). No infectious organisms or neoplastic cells were observed within the smears. Given the numeric data and the cytologic findings, the effusion was classified as an exudate (Question 1).
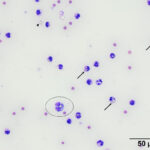 |
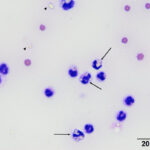 |
The perforated gastric ulcer and suppurative peritonitis explained the inflammatory leukogram, which had likely been there for several days, given the degree of neutrophilia and monocytosis (supporting a bone marrow response with granulocytic and monocytic hyperplasia). The peritonitis likely preceded but was exacerbated by the barium, which would induce a sterile chemical inflammatory response. The thrombocytopenia could have been due to concurrent disseminated intravascular coagulation, triggered by the severe inflammation, although platelet aggregation or activation could also be occurring. The mild hypoalbuminemia was attributed to a negative acute phase response (downregulated production in response to inflammation) and possible losses into the exudative effusion. The hyperphosophatemia was attributed to the azotemia, which was likely prerenal, although acute kidney injury cannot be ruled out.
Follow up
With the finding of barium in the peritoneal effusion and the previous suspicion of a septic peritonitis, a gastrointestinal rupture was considered likely and exploratory surgery was performed to find the site of leakage. A perforated pyloric ulcer was found, which had progressed to a 360-degree transection of the small intestine from the stomach, with leakage of intestinal contents into the abdomen. Barium staining of the omentum was noted along with moderate generalized peritonitis. The edges of the ruptured stomach and pylorus were debrided and anastomosed with a simple interrupted pattern. A portion of the barium-stained omentum was resected. The patient recovered from general anesthesia, however, despite being maintained on vasopressive drugs, the patient developed severe hypotension, and eventually went into cardiac arrest. The animal could not be resuscitated.
Discussion
Radiographic contrast studies are a commonly used diagnostic tool to evaluate the gastrointestinal tract (GIT), urogenital tract, and the central nervous system in veterinary and human medicine. These studies can help reveal filling or emptying defects, obstructive lesions, foreign objects, neoplasms, cysts, or other tissue defects or injuries. Commonly used contrast media include barium sulfate suspensions, iodinated products (ionic or nonionic), and barium-impregnated polyethylene spheres (BIPS).1
Barium sulfate suspensions are frequently used in positive-contrast studies involving the GIT. Medical grade barium sulfate suspensions consist of a white crystalline powder that usually has a particle size of < 1 µm and is processed to reduce the presence of impurities.2 These products are considered to be nontoxic due to limited water solubility, which is in contrast to soluble barium salts, which are highly toxic.2 Contraindications for the use of positive-contrast studies in the GIT, especially with using barium sulfate suspensions, include cases with obvious radiographic evidence of bowel obstructions or GIT perforation (i.e., free gas within the peritoneal cavity).1 Leakage of barium sulfate along with GIT contents, through pre-existing perforations, can exacerbate the exudation of fluid and albumin into the peritoneal cavity and can result in hypovolemia and hypoalbuminemia.3 This can lead to acute vascular shock and cardiac arrest, as seen in this case. In addition, fibrinous adhesions develop within several hours after the initial leakage.3 Bacteria and GIT contents can become entrapped within the fibrinous adhesion and lead to an infiltration of leukocytes that can form abscesses and eventually granulomas.3,4 Macrophages, with or without neutrophils, containing phagocytosed barium sulfate granules can be seen cytologically within peritoneal fluids and embedded within granulomas in histologic sections.2,3 Similar findings can be seen in respiratory fluid samples (e.g., transtracheal wash fluid) if a patient aspirates barium sulfate suspension into the respiratory tract after administration or if there was accidental tracheal administration of a barium sulfate suspension.5 As seen in this case, treatment of barium peritonitis is through exploratory surgery with the goal of removing the free and intracellular barium via saline flushing of the peritoneal cavity, gentle surgical sponge mopping up of barium from serosal surfaces, and excision of barium-stained omentum, if possible. Medications and conditions that increase the risk for developing GIT ulcerations and perforations include NSAID administration, hepatic disease, various systemic disease processes (e.g., disseminated neoplasia, septicemia, uremia, and disseminated intravascular coagulation), stress, and mastocytosis.6
Administration of NSAIDs is a frequently encountered risk factor for GIT ulceration. The pathogenesis of NSAID-induced injury may be multifactorial and could involve direct topical irritation that leads to increased epithelial permeability, enterohepatic recirculation of the NSAID or its metabolites, increased enteric bacterial numbers, and increased numbers of infiltrating neutrophils in response to the initial tissue injury.7 Enterohepatic recirculation refers to the mechanism by which products (i.e., bile acids, drugs, or other substances) produced or processed by the liver are excreted into the biliary system, expelled into the small intestine, absorbed by enterocytes, and recycled back to the liver. This process can allow for repeat exposure of the small intestinal mucosa to NSAIDs and their metabolites, which can worsen mucosal injury.7 Reports have also indicated that the combination of a NSAID and bile can produce worse intestinal damage than an NSAID alone.7 Meloxicam has been reported to be predominantly eliminated from the body through the biliary system, making enterohepatic recirculation a possible mechanism for the development of GIT ulceration.7 Deracoxib (Deramaxx®) undergoes hepatic transformation into four metabolites and is excreted from the body in the feces (Deramaxx® package insert). Although the fraction of biliary excretion of this drug and its metabolites is unknown,8 enterohepatic recirculation could be a possible mechanism, as well, for GIT injury and ulceration. The administration of different NSAIDs in close proximity with or without concurrent administration of a corticosteroid has also been reported to increase the risk of GIT ulceration and perforation in dogs.6
Both Meloxicam and Deracoxib are COX-2 selective inhibitors that interfere with the production of inducible pro-inflammatory prostaglandins while sparing protective GIT prostaglandins. However, COX-2 derived prostaglandins have been reported to possibly play a role in maintaining the integrity of the intestinal mucosa and facilitating GIT ulcer healing.7 These findings suggest another mechanism for the development and progression of GIT ulceration by COX-2 selective inhibitors.7 Although NSAIDs are beneficial for the management of inflammation and pain, these medications should be administered with caution and a “wash-out” period should be incorporated when switching between different NSAIDs or starting corticosteroids.
Author: S Chu.
References
- Riedesel EA. Small Bowel. In: Thrall DE, ed Textbook of Veterinary Diagnostic Radiology. 7th ed. St. Louis, MO: Elsevier; 2018: 926-954.
- Renschler J, Tarigo J, Neel J, Grindem C. What is your diagnosis? Particulate material in peritoneal fluid from a dog. Vet Clin Pathol. 2008;37(1):129-132.
- Ko JJ, Mann FA. Barium Peritonitis in Small Animals. J Vet Med Sci. 2014;76(5):621-628.
- Matiasovic M, Nutt A, Parsons K, Doran I. Barium peritonitis with abdominal abscessation. J Small Anim Pract. 2018;59(8):514-514.
- Núñez-Ochoa L, Desnoyers M, Lecuyer M. What Is Your Diagnosis? Vet Clin Pathol. 1993;22(4):122-123.
- Lascelles BDX, Blikslager AT, Fox SM, Reece D. Gastrointestinal tract perforation in dogs treated with a selective cyclooxygenase-2 inhibitor: 29 cases (2002–2003). J Am Vet Med Assoc. 2005;227(7):1112-1117.
- Reed S. Nonsteroidal anti-inflammatory drug-induced duodenal ulceration and perforation in a mature rottweiler. Can Vet J. 2002;43(12):971-972.
- Case JB, Fick JL, Rooney MB. Proximal Duodenal Perforation in Three Dogs Following Deracoxib Administration. J Am Anim Hosp Assoc. 2010;46(4):255-258.
