Hypochromasia
Hypochromasia indicates that the red blood cells have less hemoglobin than normal and the term, hypochromasia, is used in two contexts:
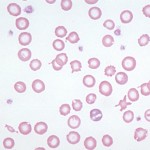
- As a descriptor of red blood cells on a blood smear: Here it refers to the appearance of red blood cells with a thin rim of cytoplasm (due to less hemoglobin) resulting in increased central pallor.
- To denote an mean corpuscular hemaglobin concentration (MCHC) below the reference interval. Hypochromasia or hypochromic red blood cell indices in this sense does not necessarily correlate with the appearance of thinner hemoglobin rims (increased central pallor) in a smear. In developing iron deficiency anemia, the appearance of hypochromasia in smears precedes a subnormal MCHC.
Hypochromasia is caused by two main mechanisms:
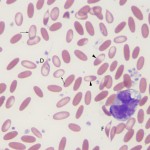
- Defective hemoglobin production:
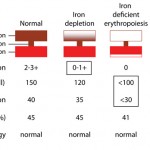
Development of iron deficiency anemia - Inherited defect: Inherited defects in hemoglobin production are because of genetic mutations that result in abnormal production of the glob in (amino acid) chains. This occurs in humans and are called thalassemias (α-thalassemia with defects in the α-chain of hemoglobin and β-thalassemia with defects in the β-chain of hemoglobin), but have not been described in animals. Interestingly, porphyries (inherited defects in synthesis of the protoporphyrin ring of hemoglobin) do not result in hypochromic anemia in affected animals.
- Iron deficiency: Since iron is an essential component of the heme group (porphyrin ring + iron), iron deficiency results in decreased hemoglobin production. Iron deficiency is most commonly caused by chronic external blood loss from the gastrointestinal tract, where slow intermittent hemorrhage (that is not readily observed by an owner) depletes iron stores, which limits erythropoiesis and results in the production of red blood cells that have less hemoglobin and are smaller than normal (the “correct” hemoglobin content is a signal for a red blood cell to stop dividing; when hemoglobin is insufficient, the red blood cell continues to divide, which each division resulting in successively smaller cells). When advanced, this results in an iron deficiency anemia, where the red blood cells are microcytic (low mean cell volume) and hypochromic (low MCHC) and are clearly hypochromic in a blood smear. Iron deficiency anemias occur more readily in young animals who have low iron stores (milk is low in iron, neonates do not have obtain iron through the placenta, and they are rapidly growing), so that any source of blood loss (e.g. severe flea infestation) can result in iron deficiency. Iron deficiency anemia can also result from nutritional iron deficiency (or copper deficiency, see below), but this is rare in domesticated animals on commercial pet food or meat diets (which are replete in iron). Young piglets used to frequently suffer from iron deficiency anemia since they could not access iron in soil (when housed intensively). This is overcome by giving them intramuscular iron injections.
- Copper deficiency: Copper is required for utilization of iron. Copper is an essential cofactor for the enzymes that allow the release of iron from stores with macrophages and uptake of iron from the gastrointestinal tract. Copper deficiency can manifest as an iron deficiency anemia. Although plasma/serum copper levels can be measured (see related links below), diagnosis of copper deficiency requires measurement of liver copper stores, because plasma copper levels do not always correlate to total body stores. We have seen microcytic hypochromic anemia in Musk Ox from a dietary copper deficiency. In ruminants, copper can be deficient in the diet or zinc or molybdenum excess can impair absorption or availability of copper, resulting in a secondary copper deficiency.
- Other deficiencies: Pyridoxal or vitamin B6 is an essential cofactor for the enzyme δ-aminolevulinic acid dehydrogenase (ALAD), which catalyzes the first step of the heme synthesis pathway, i.e. the conversion of δ-aminolevulinic acid to porphobilinogen. Deficiencies of vitamin B6 can result in microcytic anemia in pigs but the anemia is not typically hypochromic.
- Inhibition of hemoglobin production: Inhibition of heme synthesis can result in an iron deficiency anemia, particularly if the inhibition is chronic. The most common cause of heme synthesis inhibition is lead poisoning. Lead binds to sulfhydryl groups of enzymes and inhibits the activity of the following enzymes that are involved in heme synthesis: ALAD and ferrochetolase (which catalyzes the formation of heme by joining the iron group with protoporphyrin IX). Since iron is not being used for heme synthesis, it accumulates in the developing red blood cells, forming siderocytes. In chronic lead poisoning, a “relative” iron deficiency can result (relative because the iron is in the body, it just cannot be used), resulting in a microcytic hypochromic anemia. However, classic lead poisoning usually results in a normocytic normochromic anemia.
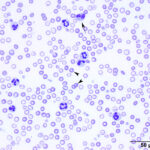
For all practical purposes, true hypochromasia in common domestic animal species occurs only in the context of advanced iron deficiency anemia.
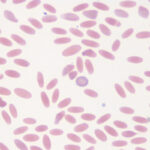
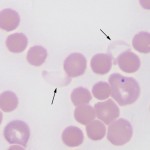
It is recognized most frequently in dogs and camelids. In both species, the iron deficiency is attributed to chronic gastrointestinal hemorrhage. In the dog, hemorrhage from colonic vascular ectasia and bleeding ulcers (e.g. corticosteroid or non-steroidal anti-inflammatory drug treatment or gastrointestinal tumors are common causes of iron deficiency anemia. In the camelid, iron deficiency anemia has been attributed to gastrointestinal blood loss associated with the blood-sucking strongyle parasite, Haemonchus contortus (“barber’s pole” worm). Hypochromasia in advanced iron deficiency is accompanied by erythrocyte shape abnormalities suggesting fragmentation (schistocytes, keratocytes, acanthocytes) in the dog. This may result from diminished deformability of the iron deficient cells, because they are thought to be more rigid than normal (mechanically fragile). In camelids, fusiform (acuminocytes) and tear-dropped shaped (dacryocytes) red blood cells are common concurrent findings in iron deficient anemia (see image above), however the mechanism of formation of these poikilocytes is unknown.
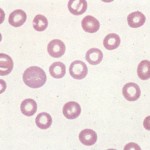
Hypochromic red blood cells must be distinguished from torocytes, an artifactual red blood cell change that mimics hypochromasia. Torocytes have no diagnostic relevance, other than that they could be mis-identified as hypochromic red blood cells, leading to an erroneous diagnosis of iron deficiency.
Polychromasia
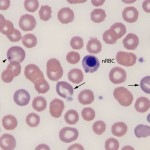
Polychromasia is a feature of immature anucleate erythrocytes (which are also aggregate reticulocytes) in the blood. The immature RBC are blue because they contain moderate to large amounts of RNA (ribosomes, polyribosomes) which offsets the red of hemoglobin, imparting a purple color to the cells. In many species, once the cell reaches the reticulocyte stage, it remains in the bone marrow for about 2 days, then is released to complete its maturation by losing its RNA and some of its surface membrane while it circulates. This is usually accomplished in the spleen. Consequently, low numbers of polychromatophils are seen in healthy dogs (< 1.5% reticulocytes). Immature a nucleate RBC with RNA are not released from the marrow in normal horses and ruminants. In all species, other than the horse, the degree of polychromasia in a blood smear is a good guide as to whether the bone marrow is responding (by releasing immature anucleate RBC) to an anemia, i.e. the anemia is regenerative if there are sufficient polychromatophils present. The horse (and other equine) is an exception as they do not typically release polychromatophils in response to an anemia (they release cells that are larger than normal, called macrocytes). In dogs and cats, the number of reticulocytes can be quantified as a percentage or an absolute count.
There is often confusion as to the meaning of the terms, reticulocytes and polychromatophils (polychromatophilic RBC).
Reticulocytes
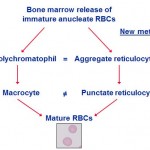
Reticulocytes are immature anucleate erythrocytes containing RNA that stain blue with dyes such as non-methylene blue (NMB) and oxazine-750 or fluoresce with dyes that bind to RNA (e.g. thiazole orange). When they contain moderate or large amounts of RNA, they are called aggregate reticulocytes and stain purple in a Wright’s or rapid (e.g. Diff-quik) stain, but if they only contain a little RNA, they are called punctate reticulocytes, they will not stain purple and will be red. Thus, there are always more reticulocytes than polychromatophils (polychromatophils are only aggregate reticulocytes and are not punctate reticulocytes). This distinction matters in cats, where only aggregate reticulocytes (or polychromatophils) are counted as part of the regenerative response.
Polychromatophils
Polychromatophils are reticulocytes that do contain sufficient RNA to stain blue-purple with Wright’s stain. They consist of the most immature reticulocytes (i.e. aggregate reticulocytes), as they contain the most RNA. All polychromatophils are reticulocytes, however not all reticulocytes are polychromatophils on a Wright’s stained blood smear, as mentioned above)
Assessment of reticulocytes help determine if an anemic patient has a healthy marrow response to the anemia as would be indicated by increased numbers of young red cells produced to replace lost red cells. Interpretation of the reticulocyte count differs with species.
Ghost red blood cells
Red blood cell ghosts represent cells that have ruptured in the circulation, losing their hemoglobin. The remaining red blood cell membranes are then seen as “ghosts”. Ghost red blood cells represent red blood cell lysis (hemolysis). This can be a true in vivo finding or an in vitro artifact.
-
- In vitro artifact: Low numbers of ghost red blood cells can be seen in any smear, if the cells are ruptured during smear preparation. Lipemic samples (e.g. animals not fasted before sample collection or animals with hyperlipidemic states) are more prone to RBC lysis. If samples are collected or handled incorrectly (e.g. frozen), red blood cells can lyse in the tube, resulting in many ghost cells. This must be distinguished from true in vivo hemolysis, which is of pathologic relevance. This can be accomplished by evaluating the animal for a cause of intravascular hemolysis (see below) or documenting hemoglobinuria (which should accompany true intravascular hemolysis). Hemoglobin from ruptured cells (whether from in vitro or in vivo hemolysis) is a major interference, affecting the results of many clinical pathologic tests and confounding interpretation.
- In vivo intravascular hemolysis: There are various causes of intravascular hemolysis in animals, including oxidant injury and erythroparasites. These can lyse red blood cells in the circulation, resulting in hemoglobinemia and hemoglobinuria. Because hemoglobin can cause injury to renal tubules (either direct cytotoxicity or through binding nitric oxide, causing hypoxia-related tubular necrosis), hemoglobinemia and hemoglobinuria have major pathologic consequences, including acute renal failure. Therefore, distinction between artifactual (in the tube) hemolysis and real in vivo intravascular hemolysis is crucial. This requires assessment of the patient (for a cause of intravascular hemolysis), knowledge of sample collection (a difficult collection with shearing of blood may hemolyze red blood cells) and handling (submitting samples in the dead of winter with no protection from cold), and documentation of hemoglobinuria (must be distinguished from hematuria), which will only occur with in vivo hemolysis. Note, that some animals with acute intravascular hemolysis may have normal hematocrits, so the presence or absence of anemia should not be relied upon to distinguish between true in vivo hemolysis and an artifact. Causes of intravascular hemolysis include:
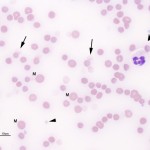
Ghost cells in a horse with oxidant injury due to red maple leaf toxicity. - Oxidants: Zinc pennies in dogs, onions in dogs and cats (onion-based baby food), naphthalene moth balls (dogs), skunk musk (dogs, pandas), red maple leaves (horses), copper poisoning (sheep). Eccentrocytes and Heinz bodies support the presence of oxidant injury to RBC in this setting. Heinz bodies can be readily identified in ghost RBC.Parasites: Babesia species.
- Bacteria: Clostridial toxins, Leptospira.
- Venoms: Snake venoms, bee stings (mellitin).
- Metabolic conditions: Acute liver failure (horses), hypophosphatemia (dogs, cats, cattle)
- Drugs: DMSO, phenothiazine drenches (horses), vitamin K (dogs), propofol (dogs).
