There are many different causes, which can be separated mechanistically as done below. A retrospective study of anemia in 456 dogs (excluding those with acute blood loss anemia) showed that the most frequent causes of anemia were inflammatory disease and cancer-associated anemia (which could be multifactorial in origin and the cohort could have included cases with splenic tumor-related hemoperitoneum), which comprised 62% of the total cases, followed by immune-mediated hemolytic anemia (IMHA, 13% of cases). Anemia associated with renal disease and endocrinopathies were less frequent (8 and 3%, respectively). Anemia of inflammatory disease, cancer- or endocrinopathy-associated anemia, and anemia due to renal disease were typically mild to moderate (20-36% hematocrit [HCT] in >90% of cases). More severe anemias (<20% HCT) were seen more frequently in IMHA (Chervier et al 2012).
Immune-mediated hemolytic anemia
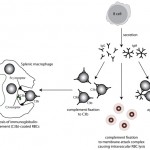
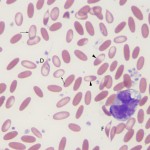
Immune-mediated hemolytic anemia (IMHA) is most common in the dog (Garden et al 2019) but can occur in other species, including cats (Swann et al 2016). Classically these are acutely developing regenerative anemias, in which RBC are coated with antibody (IgG usually, but also IgM and IgA) and/or complement (C3b) and are prematurely removed from circulation by macrophages in the spleen (mostly), liver and bone marrow (extravascular hemolysis). In addition, eryptosis (programmed cell death in red blood cells with removal of phosphatidylserine (PS)-expressing RBCs by macrophages, which contain receptors for PS) may contribute to increased RBC destruction in IMHA (in one study, 11 dogs with IMHA had up to 5.5% PS-expressing RBCs [Lucidi et al 2021], although binding of PS-enriched platelet-derived microparticles could be responsible for this finding, versus eryptosis). In some animals, complement fixation by antibodies (particularly IgM, but also subtypes of IgG) can result in a concurrent intravascular hemolysis (see image to the right) due to fixation of the membrane attack complex (complement components 6-9) resulting in membrane lysis. In humans, intravascular hemolysis can destroy far more cells than extravascular hemolysis, partially explaining the more severe clinical manifestation of IMHA when there is concurrent intravascular hemolysis (Barcellini et al 2020). Free hemoglobin is also toxic to the kidney (directly to renal tubules and indirectly via scavenging nitric oxide, causing renal vasoconstriction) and potentially other cells, such as endothelial cells. IMHA can be primary or non-associative or secondary or associative due to drugs (e.g. penicillin in horses) or erythroparasites (e.g. Babesia sp., Mycoplasma haemofelis), with primary or non-associative causes being more common (Woodward and White 2020). Certain dog breeds have a higher incidence of IMHA including Cocker Spaniels and Old English Sheepdogs. The antigens on the RBCs that are responsible for antibody binding in animals are not known. There is evidence that IMHA is associated with T cell dysregulation and production of auto-reactive T helper (Th17) cells and/or suppression of regulatory T cells. Cytokine abnormalities can also help skew the immune response to auto-reactivity, but it is not known if cytokine dysregulation in IMHA is primary or secondary to the inflammation associated with the disorder. In cases of primary non-associative IMHA, antigens are thought to be normally expressed on RBC surfaces, whereas in secondary associative IMHA, the primary cause may bind to RBCs (either directly or via immune-complex formation) or foreign proteins may resemble RBC self antigens triggering an antibody response (called “molecular mimicry”). In lymphoid neoplasms in human patients, auto-reactive B cells may be responsible for IMHA (so-called “emergence of forbidden clones”) (Barcellini et al 2020).
The following features in a blood smear help us identify an IMHA (reviewed by McNeill et al 2019):
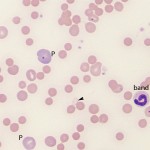
- Moderate to many spherocytes: This characterizes most IMHA in dogs (spherocytes are harder to identify in other species). Note that small numbers of spherocytes may be seen in disorders other than IMHA, including transfusion of stored RBCs, fragmentation injury, and abnormal macrophage function (e.g. hemophagocytic syndrome, hemophagocytic variant of histiocytic sarcoma). Therefore spherocytes do not always indicate an IMHA.
- Agglutination. This should be distinguished from rouleaux formation by dilution with saline (using a ratio of 1:4 to 1:10 RBC to saline – rouleaux should disperse whereas agglutination is usually still present on slide agglutination tests or microscopic examination of the diluted blood. Ideally, persistent agglutination should be confirmed after washing RBC, however this washing step is rarely done in clinical practice.
- Positive direct Coombs test. A direct Coombs test assesses for the presence of immunoglobulin (IgM, IgG) or complement (C3b, C3d) on RBC using a Coombs reagent, which consists of species-specific anti-Ig and/or anti-C3. A positive Coombs test is supportive evidence of IMHA, but false positives and negatives do occur (Wardrop 2012). A Coombs test may not be indicated if there is clear evidence of IMHA (many spherocytes) or saline agglutination is persistent after washing (Garden et al 2019, MacNeill et al 2019).
Other supportive or concurrent clinical pathologic findings in IMHA dogs are (these are less characterized in other species):
- Thrombocytopenia (mild to moderate mostly, sometimes severe): This can be a common concurrent finding, which in low numbers of dogs is also immune-mediated (combined immune-mediated hemolytic anemia and thrombocytopenia is called Evan’s syndrome). Other contributing causes could be splenic sequestration and consumption with coagulation activation or platelet activation, potentially due to extracellular DNA or DNA-histone complexes (see below).
- Inflammatory leukogram: Leukocytosis due to a neutrophilia with a left shift and monocytosis in over half of dogs with IMHA. Inflammatory cytokines and positive acute phase proteins are also increased in dogs with IMHA (McNeill et al 2019).
- Biochemical findings: Increases in liver leakage enzymes (ALT, AST in small animals and GLDH, SDH in large animals) may be seen due to hypoxic-induced damage to the liver. Due to hemolytic process (extravascular or intravascular), there may be a hyperbilirubinemia due to increased unconjugated or indirect bilirubin produced from heme degradation within macrophages (phagocytized RBCs or hemoglobin-haptoglobin complexes). However, many dogs with IMHA develop concurrent cholestasis, with increases in direct conjugated or direct bilirubin that may dominate (with concurrent excessive bilirubinuria). High iron and % saturation of transferrin are often seen due to increased RBC turnover.
- Hemostatic abnormalities: Many dogs (28-80%) have evidence of non-overt or overt disseminated intravascular coagulation, with high concentrations of D-dimer and fibrinogen concentration in the former and concurrent prolonged coagulation times (APTT>PT) in the latter (Carr et al 2002, MacNeill et al 2019). Dogs with IMHA are predisposed to thrombosis, particularly in pulmonary arteries (Carr et al 2002, DeLaforcade et al 2019), and hypoxic injury to tissues may contribute to hyperlactatemia and organ injury evident in many patients (MacNeill et al 2019). Hypocoagulable dogs (dogs in the hemorrhagic phase of overt DIC) have a poorer prognosis in IMHA. Neutrophil extracellular traps and extracellular DNA and histone-DNA complexes are increased in dogs with IMHA (Jeffrey et al 2015, Lawson et al 2018). These can potentially trigger coagulation via activating platelets and factor XII of the contact and intrinsic pathway of coagulation. Upregulation of tissue factor on monocytes and other cells or endothelial injury exposing tissue factor would also trigger DIC in dogs with IMHA by activating factor VII (Kidd et al 2015).
- Evidence of intravascular hemolysis: If there is an intravascular hemolytic component to the anemia (from the membrane attack complex of complement binding to RBC membranes), hemoglobinemia and hemoglobinuria (red urine with a positive result for heme on a urinary dipstick but no RBC in the urine sediment) will be seen. Ghost RBC may be identified in smears, but these cells can be seen with any cause of intravascular hemolysis (e.g. oxidant injury) and are also (and more commonly) an artifact of smear preparation or sample collection, handling and storage.
The American College of Veterinary Internal Medicine has established a consensus statement for diagnosis of IMHA and testing for associative conditions (Garden et al 2019). The diagnostic algorithm is first based on evidence of immune-mediated destruction based on two or more of the following: 1) Moderate to many spherocytes (dogs only), 2) Positive saline agglutination tests without washing, and 3) Detection of RBC-bound immunoglobulin or complement with Coombs or flow cytometric testing or a persistent positive saline agglutination test after washing RBCs. The second tier of evidence consists of documentation of 1 or more of the following signs of hemolysis via: 1) Hyperbilirubinemia (which will only occur if bilirubin production exceeds liver uptake or cholestasis developes), excessive bilirubinuria (indicating cholestasis) or icterus without evidence of hepatic disease or biliary obstruction/rupture or sepsis, 2) Hemoglobinemia, 3) Hemoglobinuria, or 4) Ghost RBCs (not specific for intravascular hemolysis). However, surveys show that there is variability in the tests used to diagnose IMHA (McNeill et al 2019).
Causes of associative IMHA:
- Neoplasia: Lymphoma in horses (McGovern et al 2011), lymphoma/lymphoid leukemia in dogs and cats (intermediate to low level of evidence, Garden et al 2019).
- Infections: Post-Streptococcal infections (purpura hemorrhagica is an immune complex vasculitis that is secondary to infection with Streptococcus equi var equi, the causative organism of Strangles), Clostridium perfringens (horses [Reef 1983, Weiss and Moritz 2003]), Babesiosis in dogs and cats (intermediate to high level of evidence, Garden et al 2019), viral infections (FeLV in cats, Garden et al 2019), Mycoplasma haemofelis (Garden et al 2019), Anaplasmosis (intermediate to low level of evidence in dogs and cats, Garden et al 2019).
- Drugs: Penicillin (Blue et al 1987) and trimethroprim-sulfur (Thomas and Livesey 1998) have been associated with a drug-induced hemolytic anemia in horses. Cefazedone and propylthiouracil are associated with IMHA in dogs and cats, respectively (Garden et al 2019).
- Alloimmune: Neonatal isoerythrolysis (NI) (Polkes et al 2008) and incompatible blood transfusions. This is when there are blood group incompatibilities between a mother and offspring (NI) or between a recipient and a donor (transfusion reaction, the most serious of which is when the recipient rapidly lyses donor cells).
Non-regenerative or precursor-directed immune-mediated anemia
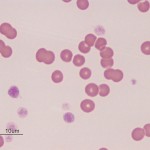
Dogs and cats with non-regenerative immune-mediated anemia (also called precursor-directed immune-mediated anemia or PIMA) usually present with severe normocytic normochromic anemia (Hcts, < 15-20%) of long duration, with normal leukocyte and platelet counts (Stokol et al 2000, Weiss et al 2008, Black et al 2016, Lucidi et al 2017). Up to 50% of cases in one study of 15 cats with NRIMA and PRCA were concurrently neutropenic, thrombocytopenic or pancytopenic (Black et al 2016). Due to the long duration, affected animals often do not display clinical signs of hypoxia that are typically associated with such severe anemias. There is no or insufficient evidence of regeneration in blood and RBC morphology is usually normal, although some dogs may have partial spherocytes in peripheral blood. They are usually Coombs negative, although a few dogs may be Coombs positive (often weak reactions, i.e. only present at low dilutions of the Coombs reagent). Biochemical panels are typically normal, except for hyperferremia and increased transferrin saturation. Some dogs may concurrently have extravascular hemolysis and associated hematologic (many spherocytes, strong positive Coombs reaction) and biochemical findings (increased total and indirect bilirubin concentrations). Diagnosis requires bone marrow aspiration and ruling out other causes of non-regeneration (e.g. neoplasia). Bone marrow aspiration often reveals an erythroid hyperplasia, with increased marrow iron (dogs) and a concurrent mild reactive lymphocytosis and plasmacytosis, compatible with an ineffective erythropoiesis. There may be a left-shifted maturation sequence in erythroid progenitors (more immature than mature forms) and erythrophagia (of precursors, also called rubriphagocytosis, or mature erythrocytes). However, some dogs can have an erythroid hypoplasia, if earlier precursors are targeted (Lucidi et al 2017). Dogs may have secondary reticulin and collagen myelofibrosis, which may prevent a good bone marrow aspirate, necessitating a core biopsy. Secondary myelofibrosis is rare in cats and mild (Black et al 2016). A study in 17 dogs with PIMA showed antibody binding to nucleated RBC precursors in the marrow (>20% cells with bound IgG) in low numbers of dogs (5/17, 29%) and a similar number of precursors were expressing phosphatidylserine (20-80% of cells). This data suggests that destruction could be cell-mediated versus Ig-mediated in this bone marrow centric condition (Lucidi et al 2021). The anemia is presumed immune-mediated as it responds to immunosuppressive therapy, such as a combination of azathioprine in dogs (which can suppress the bone marrow in some dogs), cyclosporine (dogs and cats), mycophenolate and high doses of prednisolone (dogs and cats). A response (increased reticulocytes, hematocrit) is usually observed within 2-4 weeks of treatment. Long-term therapy may be required; do not taper therapy too rapidly, since the disease can recur and become refractory to therapy (Stokol et al 2000, Black et al 2016). Both NRIMA/PIMA can be considered variants of immune-mediated anemia (see image below).
Pure red cell aplasia
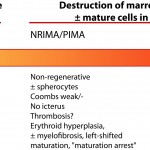
Pure red cell aplasia (PRCA) is a more severe form of PIMA, characterized by the absence of identifiable erythroid precursors in marrow (<5% of marrow cells). It has been diagnosed rarely in dogs, cats (Stokol et al 2000, Stokol et al 2009, Weiss et al 2008, Black et al 2016) and ferrets (Malka et al 2010, Kaye et al 2015). Cats are usually young (< 3 years of age) and negative on testing for feline leukemia virus DNA or antigen. Once again, this disorder responds to aggressive immunosuppressive therapy. Tapering of drugs too rapidly may lead to recurrence, which becomes refractory to further treatment. Pure red cell aplasia has also been documented in dogs, horses and cats as a direct consequence of recombinant human erythropoietin administration (Piercy et al 1998, Randolph et al 2004 [cats], Randolph et al 2004 [dogs]). It does resolve after drug withdrawal, however the associated anemia can be profound and protracted. In one study of 15 cats (8 NRIMA, 7 PRCA), affected animals were treated with immunosuppressive doses of steroids, with or without chlorambucil or cyclosporine (typically cats were given the latter drugs when they did not respond to therapy with steroids alone or if steroid treatment was contraindicated due to comorbidities). A response to treatment was seen within 12 days (up to 42 days) in 11 cats, with an overall mortality rate of 27%. Relapses occurred in 3 of the 11 treated cats within 2-9 months, usually with tapering of therapy (Black et al 2016).
Oxidant-induced hemolytic anemia
Oxidant injury to RBC is caused by various plants, chemicals, minerals, and drugs (see below). Animals with inherited deficiencies in oxidant protection pathways, e.g. glucose-6-phosphate dehydrogenase deficiency in horses, are predisposed to oxidant-induced hemolytic anemias (see below for inherited defects). Many oxidants (e.g. red maple, zinc, copper) cause intravascular as well as extravascular hemolysis. Note that although eccentrocytes and Heinz bodies characterize oxidant injury (and keratocytes), these red blood cell changes are not seen with all oxidants or in all species. It depends on whether the oxidative injury is affecting the RBC membrane (to produce eccentrocytes) or hemoglobin (to produce Heinz bodies).
- Plants: Onions (dogs, cats), brassica species (ruminants; causative compound is thought to be S-methylcystein sulfoxide, which is converted to the oxidant dimethyl disulfide in the rumen [Prache 1994), wilted red maple leaves (camelids [DeWitt et al 2004], horses [Alward et al 2006]), Pistacia leaves (horses) (Bozorgmanesh et al 2015), nitrate-containing plants (ruminants). With red maple and Pistacia species (plant of the cashew family, one of which produces pistachios), a key toxic compound is thought to be gallic acid, which accumulates in wilted leaves of red maple and is concentrated in the seed pods of Pistachia (Walter et al 2014). The gallic acid is converted to an even more potent anti-oxidant, pyrogallic acid, in the ileal lumen by intestinal microbes. Tannic acid is thought to be an additional oxidant in red maple leaves and is also converted to gallic acid and then pyrogallic acid by intestinal microbes (Agrawal et al 2013).
- Minerals: Zinc (dogs ingesting pennies minted after 1982), copper (particularly sheep due to their low copper requirements compared to cattle, rare in camelids, dogs with inherited diseases causing copper accumulation, e.g. Bedlington terriers).
- Chemicals: Napthalene moth balls (dogs), skunk musk (dogs>cats) (Fierro et al 2013).
- Drugs: Acetaminophen (cats), vitamin K (dogs).
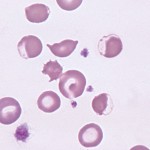
The following features in a blood smear help us identify oxidant injury:
- Eccentrocytes and pyknocytes: Eccentrocytes are RBC with an “eccentric” area of central pallor caused by crosslinking of oxidized RBC membranes. When eccentrocytes are partially phagocytized by macrophages, spherical small RBC called pyknocytes are formed. These resemble spherocytes in blood smears, except that they have a small tag of membrane (often only discernable by electron microscopy).
- Heinz bodies: These are the result of oxidation of the globin chains of hemoglobin, which then precipitates. These are most easily identified in new methylene blue-stained blood smears.
- Keratocytes
- Methemoglobinemia: This is due to oxidation of the iron molecule within hemoglobin and may occur with some oxidants. It results in a brown discoloration of the plasma and mucous membranes.
Note, when these RBC morphologic abnormalities (eccentrocytes, pyknocytes, keratocytes) are seen in low numbers in an anemic animal, they are unlikely to be the cause of the anemia. All they mean is that there is oxidant injury to RBC and, even though affected RBC will have decreased lifespan, it does not mean the animal has an oxidant-induced hemolytic anemia. For example, low numbers of eccentrocytes are seen in dogs with various diseases (Caldin et al 2005). We have seen numerous single small Heinz bodies in non-anemic goats (presumptive underlying selenium or vitamin E deficiency). Non-anemic cats often have small or so-called “endogenous” Heinz bodies, because their hemoglobin is more susceptible to oxidant injury than other species. Increased numbers of endogenous Heinz bodies are seen in cats with many diseases, particularly lymphoma, hyperthyroidism and diabetes mellitus (Christopher et al 1989, Christopher et al 1995). Although these cats are often not anemic, their RBC lifespan is reduced, i.e. the affected RBC are being hemolyzed or prematurely removed from circulation (Christopher et al 1989). In anemic cats and goats with these “endogenous” Heinz bodies or dogs with low to moderate numbers of eccentrocytes without Heinz bodies, the anemia is more likely that the underlying disease causing the oxidative injury is also causing the anemia (which is frequently due to bone marrow suppression or a hemolytic process from inflammatory or chronic disease). It is when we see moderate to high numbers of these oxidant changes in RBCs (very large or multiple Heinz bodies in cats, eccentrocytes with Heinz bodies in a dog, eccentrocytes or Heinz bodies in a horse) that we attribute an anemia to oxidative injury.
Fragmentation hemolytic anemia
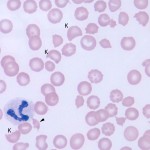
Fragmentation anemias are frequently secondary to vascular disease (e.g. vasculitis, hemangiosarcoma) or disseminated intravascular coagulation (DIC) and are also called microangiopathic hemolytic anemias, when the fragmentation is occurring secondary to small vessel disease (those conditions above). Unlike typical hemolytic anemias, fragmentation anemias can be (and frequently are) non-regenerative as cytokines associated with the primary disease concurrently suppress the bone marrow. Fragmentation injury to RBC can also occur if RBC are subject to severe heat stress (e.g. burns) or iron deficiency (RBC are thought to be mechanically fragile in this setting, see below). In these cases, the anemia is not called a microangiopathic hemolytic anemia.
The following RBC changes are seen in blood smears with fragmentation:
- Keratocytes, schistocytes, acanthocytes: Keratocytes are not specific for fragmentation but can be seen in oxidative injury as well. To determine what type of injury is causing the keratocytes, we look at the company they are keeping – if there are eccentrocytes or Heinz bodies, we would conclude it is oxidative injury. If there are acanthocytes and schistocytes, we would conclude there is fragmentation injury. If none of these other changes are seen, either type of injury could be occurring. A few spherocyte-like cells may be also be seen with fragmentation and do not indicate immune-mediated disease in this setting (they are a type of fragment).
- Thrombocytopenia: Thrombocytopenia usually accompanies fragmentation due to DIC.
Note, when the above RBC morphologic abnormalities (keratocytes, schistocytes, acanthocytes, spherocyte-like cells) are seen in low numbers in an anemic animal, they are unlikely to be the cause of the anemia, e.g. fragments in iron deficiency anemia, turbulent blood flow (e.g. portosystemic shunts), liver disease. All they mean is that there is fragmentation injury to RBC and, even though affected RBC will have decreased lifespan, it does not mean the animal has a fragmentation hemolytic anemia. It is more likely that the underlying disease (e.g. iron deficiency itself) causing the fragmentation injury is the main cause of the anemia. When there are moderate to high numbers of these RBC abnormalities, we attribute an anemia to fragmentation or consider that fragmentation is contributing to the anemia.
Iron deficiency anemia
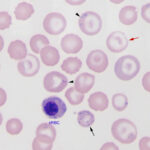
An iron deficiency anemia occurs when iron becomes limiting for erythropoiesis. Iron is an essential component of the heme group of hemoglobin and, in the absence of iron, hemoglobin cannot be produced in sufficient quantities. Total body depletion of iron (absolute or true iron deficiency) will eventually result in a microcytic hypochromic anemia and hypochromasia (decreased rim of hemoglobin resulting in increased central pallor) will be evident in red blood cells on blood smear examination (see image on the right and images below). These findings are useful clues as to the presence of an iron deficiency anemia and will typically be associated with a low iron concentration and percentage iron saturation on chemistry panels (transferrin saturation is often normal but may be increased). Documentation of iron deficiency without hematologic evidence of such (hypochromasia in blood smears, low MCV, low MCHC) is difficult because serum iron concentrations are not the best surrogate of iron stores and ferritin, the iron storage protein, can be increased by inflammation. Newer sensitive markers, such as the hemoglobin content of reticulocytes (newly formed cells, e.g. CHretic and percentage of reticulocytes with low hemoglobin [% CHretic]) can be helpful in identifying iron restricted erythropoiesis before changes in the traditional RBC indices of MCV and MCHC or even the development of anemia. However, changes in these tests are not unique to an absolute iron deficiency and may be seen in dogs with other conditions (see below) and dogs with inherited microcytosis (Schaefer and Stokol 2015). We do not usually perform the “gold standard”tests of measuring tissue iron, such as bone marrow aspirates or liver biopsies for the sole purpose of assessing body iron stores.
Because of an ample storage pool and strict conservation of iron by the body, absolute iron deficiency in adult animals is usually caused by chronic external blood loss rather than by inadequate dietary iron alone (Harvey et al 1982). Nursing animals and young, rapidly growing animals develop iron deficiency more easily than adults on an iron-replete diet, particularly if there is a concurrent source of external blood loss: iron stores in young animals tend to be marginal, milk is low in iron, and rapid growth demands expansion of blood volume. For example, puppies and kittens heavily infested with fleas or hookworms (sources of external blood loss) may be concurrently iron deficient. Baby pigs and calves may, however, develop iron deficiency without chronic external blood loss. In adult animals, other causes of chronic external blood loss become more important (e.g., bleeding from tumors in the gastrointestinal tract, coagulopathies with bleeding from gastrointestinal or urinary tracts, etc.). Note that iron deficiency anemia is exceedingly rare in horses (Brommer et al 2001, Fleming et al 2006). For more on physiology of iron, refer to the iron metabolism overview. It is important to recognize an iron deficiency anemia because iron supplementation will be required to help correct the anemia, along with treatment of the cause of the blood loss.
Experimentally, iron deficiency can be induced by feeding an iron-deficient diet, particularly to young animals, or by phlebotomy. In one study of nutritional iron deficiency in 4 month old Beagles (who had low body stores of iron to begin with), it took about 6 weeks for the hematocrit to drop below 40% in 4/7 puppies, with microcytosis (MCV <65 fL) being seen in 6/7 dogs by 8-9 weeks. A low MCHC (<33 g/dL) was seen in 3/7 dogs at 8-9 weeks (Fry and Kirk 2006). This data shows how long it takes to develop iron deficiency anemia with nutritional deficiency, even when iron stores are low to begin with. Serum iron (<90 ug/dL) and percentage iron saturation (<20%) were decreased in 5-6/7 dogs at 4-5 weeks, whereas total iron-binding capacity did not increase above 450 ug/dL during this time (i.e. would be within reference intervals). Phlebotomy-induced blood loss (removal of large volumes of blood) resulted in an anemia (hematocrit <25%) in 3-8 days in 2 adult llamas, whereas the MCV and MCHC took longer to decrease (the MCV decreased by 2-4 fL within 8-10 weeks; the MCHC decreased below 40 g/dL by 5 weeks). Hypochromasia was evident in red blood cells in blood smear when the MCHC decreased below 40 g/dL. Serum iron and percentage saturation was low at 5 weeks (iron: 31-38 ug/dL; % saturation: 13%) (Morin et al 1993). The study in young beagles showed that the MCV continued to drop and MCHC remained low in the dogs even after reinstitution of an iron-replete diet or intramuscular iron dextran injections. Injection of iron resulted in better resolution of hematologic changes than dietary adjustment. The study also showed that in individual dogs, decreases in MCV preceded those of MCHC and that reticulocyte indices of hemoglobin content (CHretic and % low CHretic) were superior to MCV and MCHC for identifying iron deficiency in the dogs (Fry and Kirk 2006). However, both of these indices are only available on ADVIA hematology analyzers, which are becoming obsolete, although CHretic is available on newer hematology analyzers. The canine study did not assess red blood cell morphologic features in smears. In a study of 25 dogs with naturally acquired iron deficiency anemia, the MCV (range, 32-59 fL), MCHC (range, 23-34 mg/dL) and iron concentrations (8-60 ug/dL) were low. Hypochromasia in blood smears were evident in dogs with a low normal MCHC (i.e. can be more sensitive than the MCHC to iron deficiency anemia). Affected dogs had chronic gastrointestinal or urinary tract bleeding, associated with intestinal parasites (e.g. hookworm, whipworms), colitis, immune thrombocytopenia, and tumors (Harvey et al 1982). With iron supplementation of dogs with natural and experimental iron deficiency, the serum iron concentration and percentage saturation improves more quickly (within 5-6 days) than the MCV and MCHC, which can take months to normalize (Harvey et al 1982, Fry and Kirk 2006).
With iron deficiency anemia, the low MCHC can be readily explained by decreased hemoglobin production. However, the reason for the microcytosis is controversial. Hypotheses for the microcytosis include: 1) Immature RBC in the bone marrow stop dividing once a critical concentration of hemoglobin is reached within them. Thus, deficient hemoglobin production may result in increased cell division. With each division, RBCs become smaller, resulting in microcytic and hypochromic RBCs; 2) Iron deficiency affects enzyme activity, which may alter receptor expression on erythrocytes that govern release. If iron deficient, the erythrocytes are no longer released and when they are retained in the marrow, they continue to divide. Later-stage RBC precursors do express Lutheran adhesion molecules, but it is not known if iron deficiency decreases their expression; and 3) Iron deficiency possibly affects macrophage function in marrow causing delayed extrusion of the nucleus of erythroid progenitors so they cannot be released from marrow (a protein in erythroblasts called erythroblast macrophage protein is required for their interaction with macrophages and extrusion of their nuclei; dysfunctional interactions may impair nuclear extrusion).
Iron deficiency anemias can be regenerative or non-regenerative (Harvey et al 1982, Weiser and O’Grady 1983). The reason why some iron deficiency anemias are regenerative and others is unknown. The anemia may be regenerative if dietary iron or iron absorption is sufficient to maintain some degree of erythropoiesis or if the source of blood loss is the upper gastrointestinal tract (where iron and blood may be more likely to be reabsorbed than in the lower intestinal tract). Studies in rats show that if there is a concurrent nutritional iron deficiency with blood loss, the anemia is more likely to be non-regenerative. If dogs have concurrent disorders that suppress regeneration (inflammatory disease), these disorders will contribute to a lack of regeneration. Red blood cell lifespan is also reduced in iron deficiency anemia. This has been recently attributed to eryptosis (Kempe et al 2006). Iron-deficient RBCs show features of apoptosis (shrinkage, membrane blebbing, phosphatidylserine exposure) and are removed by splenic macrophages. Whether this also occurs in the bone marrow leading to failure of regeneration is unknown. There is also evidence of RBC fragmentation (keratocytes, acanthocytes, schistocytes) in iron deficiency in dogs (Harvey et al 1982, Weiser and O’Grady) and cats (Weiser and Kociba 1983). We have seen similar changes in ruminants with iron deficiency anemia. Camelids with iron deficiency have dacryocytes (tear drop-shaped RBCs), acuminocytes (fusiform cells) and folded erythrocytes and the pallor is often at one pole of the cell versus being central, mimicking an eccentrocyte (Morin et al 1992, Morin et al 1993) (see image gallery on camelids in the hematology album and image below). This has been attributed to increased mechanical fragility (iron deficient RBC are more rigid and more likely to fragment as they traverse through small capillaries under high shear), however oxidative injury to red blood cells is likely contributing to keratocyte formation (Yoo et al 2009) (given that antioxidant enzymes in red blood cells, such as glutathione peroxidase, contain iron).
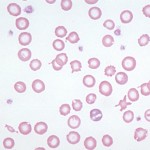
Causes of an iron deficient anemia due to absolute iron deficiency are:
- Dietary deficiency of iron: This is an uncommon cause of iron deficiency in animals. It can occur secondary to acidosis, excess vitamin C, or excess zinc. Dietary iron deficiency was suspected to be the cause of iron deficiency in foals (Brommer et al 2001, Fleming et al 2006). In one of these reports, red blood cell fragmentation was described, a very rare finding in horses (Fleming et al 2006).
- Chronic external blood loss: The most common source of blood loss is the gastrointestinal tract (e.g. bleeding ulcers, colonic ectasia, blood-sucking parasites) in adult animals. A heavy flea infestation in young animals or chronic urinary tract hemorrhage can also result in an iron deficiency anemia. Chronic external blood loss, particularly when slow or intermittent, results in a slowly developing anemia, which at first is regenerative (increased reticulocyte count). If blood loss continues long enough that body iron stores are depleted and absorption of dietary iron is inadequate to meet the demand created by increased red blood cell production, then iron becomes a limiting factor and effective erythropoiesis is decreased. The reticulocyte count falls, the anemia becomes more severe, and the MCV and MCHC decrease as the microcytic hypochromic red blood cells produced in the iron deficient state are added to peripheral blood. This is when iron deficiency anemia is most readily recognized in the laboratory. At Cornell University, we commonly see iron deficient anemia in dogs and camelids. In dogs, it is usually secondary to gastrointestinal hemorrhage secondary to bleeding ulcers or tumors or colonic ectasia (Harvey et al 1982, Fan et al., 1999). In camelids, Haemonchus contorts infection in the C3 compartment has been blamed for most cases of iron deficiency. Note, internal blood loss (e.g. hemoperitoneum) will not result in an iron deficiency because the blood (and iron) in the peritoneal cavity is recycled and not “lost” from the body (blood is “lost” from the intravascular space in this setting). If an iron deficient anemia exists in an an animal, iron panel testing is recommended, with typical results being decreased iron concentration, normal TIBC concentation and low percentage saturation. Furthermore, fecal occult blood and a urinalysis (for hematuria) would be worthwhile, unless hemorrhage is overt (i.e. melena, hematochezia, gross hematuria).
Iron deficiency anemia is usually treated by supplementation with oral iron (as ferrous sulfate). This practice has been recently questioned, because absorption of oral iron will transiently increase plasma iron, which stimulates hepcidin release, which then serves to inhibit subsequent iron absorption in the intestinal system for up to 48 hours (Schrier 2015). There are injectable forms of iron, typically iron dextran, which is given intramuscularly (it stings). Of course, giving a blood transfusion (which is not innocuous) will also provide iron as the transfused red blood cells are degraded by the monocyte-macrophage system. - Copper deficiency: Copper deficiency results in a secondary iron deficiency anemia. This is because copper is required for absorption of iron from the gastrointestinal tract and for release of iron from stores in macrophages in the body. Copper deficiency can result in a microcytic hypochromic anemia, which will not respond to iron supplementation. We have seen this in ruminants (musk ox, in particular). Copper deficiency can be secondary to excess zinc in the diet, so evaluation of the copper:iron:zinc ratios in feed rations of iron-deficient ruminants is essential.
There are also states of functional iron deficiency. A functional iron deficiency means that there are iron stores in the body (i.e. iron is not truly deficient), however the iron is not being incorporated into hemoglobin, resulting in decreased hemoglobin production or iron-restricted erythropoiesis. We see a functional iron deficiency when there is reduced heme synthesis or inhibition of heme synthesis either because hemoglobin production is being inhibited or is defective or iron is not being released in sufficient amounts to support hemoglobin production. With inhibition of heme synthesis, the iron that is not being incorporated into hemoglobin may precipitate in red blood cells, forming siderocytes (red blood cells with foci or short streaks of faint blue granules in their cytoplasm). Siderocytes will even form in a non-anemic patient, yielding a hematologic clue as to the possibility of heme synthesis inhibition. Siderocytes are, naturally, not usually seen with an absolute or true iron depletion, helping to distinguish between a true iron deficiency anemia and inhibition of heme synthesis.
Functional iron deficiency occurs under the following situations:
- Chronic lead poisoning: Lead inhibits the incorporation of iron into the porphyrin ring of heme via inhibiting heme sythetase, the enzyme that catalyzes this reaction in the last step of the heme synthesis pathway. It also decreases heme synthesis by inhibiting δ-aminolevulinic acid (delta-ALA) synthetase, the enzyme that catalyzes the first step in the heme synthesis pathway, in which succinate and glycine are combined in the mitochondria of developing erythrocytes to form d-ALA. In this case, the animal is not truly iron deficient, since iron stores are present in the animal, they just cannot be used for hemoglobin production. So instead of the iron being used for heme synthesis, it accumulates in the RBC as siderocytes (see image to the right). We can see siderocytes in blood smears before the animal with chronic lead poisoning develops an anemia that mimics iron deficiency, i.e. microcytic hypochromic red blood cells with hypochromasia on a smear.
- Vitamin B6 deficiency: Vitamin B6 (pyridoxine) is required for heme synthesis, being a cofactor for delta-ALA synthetase. Lack of vitamin B6 can result in the inability to form hemoglobin, which mimics an iron deficiency anemia, except that the iron that is not being incorporated into hemoglobin precipitates in developing and mature erythrocytes, forming sideroblasts and siderocytes, respectively. We have seen two cases of a severe microcytic hypochromic anemia with marked siderocyte formation in dogs, that resolved with vitamin B6 supplementation.
- Portosystemic shunts in dogs and cats: With congenital shunts, there is abnormal iron metabolism, with increased iron sequestration within body stores (macrophages, liver). This is also a version of functional iron deficiency and can result in microcytic normochromic or microcytic hypochromic red blood cells, with or without an anemia. Hypochromasia is not usually evident in blood smears, unless there is concurrent blood loss resulting in iron depletion (blood loss into the gastrointestinal tract can occur in animals with shunts, because of the development of intestinal varices). Serum iron concentrations and percentage saturation are frequently low in animals with shunts. In addition, reticulocyte hemoglobin-related results (CHretic, % low CHretic) in portosystemic shunts can overlap with those in iron deficiency anemia (Schaefer and Stokol 2015), making it difficult to distinguish between these conditions. Thus, the entire case information (clinical signs, other laboratory tests, e.g. biochemical evidence of a shunt, etc) should be taken into account, emphasizing that clinical pathologic data should never be interpreted in isolation. The microcytosis in animals with shunts does resolve to some extent after shunt ligation.
- Marked erythropoietic activity: Another variant of functional iron deficiency occurs in dogs with strongly regenerative anemias, where the rate of red blood cell production is thought to exceed the ability of macrophages to release iron quickly enough, resulting in iron-deficient erythropoiesis (Schaefer and Stokol 2016). Iron-deficient erythropoiesis also occurs in human given erythropoietin (Macdougall et al 1992).
- Increased hepcidin concentrations: With inflammatory disease (see anemia of inflammatory disease below), inflammatory cytokines upregulate hepcidin, which causes iron sequestration within macrophages and inhibits iron absorption. The reduced availability of iron from macrophages slows down erythropoiesis, contributing to the anemia seen in patients with inflammatory disease and can be thought of as a type of functional iron deficiency. A low iron and percentage saturation can be seen with acute inflammation but a low iron concentration and a total iron binding capacity with a normal percentage saturation is more often seen with inflammation of a few days or more duration. The low iron and percentage saturation in animals with acute inflammation is similar to that seen in animals with an absolute iron deficiency anemia, however hematologic findings are different in the two conditions, helping to distinguish them (see table below). With inflammatory disease, the anemia is typically normocytic and normochromic and hypochromasia is not evident in red blood cells in smears. In addition, there is less evidence of iron-deficient erythropoiesis in reticulocytes in anemia of inflammatory disease versus absolute iron deficiency or portosystemic shunts (Schaefer and Stokol 2015). High hepcidin concentrations are also reported in human beings with TMPRSS6 deficiency (Finberg et al 2008). TMPRSS6 is a transmembrane protein that negatively regulates hepcidin concentrations. Patients are born with a severe microcytic hypochromic anemia and have increased hepcidin concentrations. They do not respond to oral iron supplementation and have a poor response to injectable iron (Finberg et al 2008).
- Hemoglobinopathies: Abnormal production of globin chains, e.g. thalassemia in humans, also results in a microcytic hypochromic anemia that mimics iron deficiency. This has not been reported in animals but is seen in murine models. Erythropoietic protoporphyria (EPP, defect in ferrochelatase) can result in microcytic RBCs but in one case report of a presumptive congenital form of EPP in a dog, the animal was only mildly or not anemic (Kuntz et al 2020). In contrast, humans and animals, including rodent models, with congenital erythropoietic porphyria (due to uroporphyrinogen synthetase III deficiency) have a chronic hemolytic anemia as well as porphyrin accumulation with photosensitivity (Blouin et al 2020). In a rodent model, the hemolytic anemia and porphyrin accumulation was inhibited by iron chelation and inhibitors of the first enzyme in the heme synthesis pathway, delta-aminolevulinic acid synthetase (Blouin et al 2020).
References
Harvey et al 1982. Chronic iron deficiency anemia in dogs. J Am Anim Hosp Assoc 18:946-960.
Anemia of inflammatory disease
This produces a mild to moderate normocytic normochromic anemia (Chevier et al 2020) and is the most common cause of a non-regenerative anemia of this type, particularly when cancer-associated anemias are included under this category. Poikilocytes are not usually seen with anemia of inflammatory disease, but eccentrocytes may be identified in blood smears if the primary disease causes concurrent oxidant injury. Similarly, if the disease process is causing fragmentation or disseminated intravascular coagulation, RBC fragmentation may be seen. Any disease process (e.g. cancer, liver disease, gastrointestinal disease) can affect RBC production in various ways, as indicated below. This used to be called anemia of chronic disease but is now called anemia of inflammatory disease, because it is mediated by inflammatory cytokines (TNFα, IFNγ, IL-1β, and IL-6) even if there is no clinical or laboratory evidence of overt inflammation in the animal. Multiple mechanisms are responsible for the non-regenerative anemia, but the main mechanisms are decreased RBC production due to suppression of erythropoiesis, reduced iron availability, and decreased RBC lifespan (extravascular hemolysis). A bone marrow aspirate (if done) would reveal erythroid hypoplasia with increased bone marrow iron (due to hepcidin-mediated sequestration and suppression of bone marrow erythropoiesis). There may be a concurrent granulocytic hyperplasia if there is an inflammatory leukogram and cytokine-mediated skewing of hematopoiesis to myelopoiesis. Mechanisms underlying anemia of inflammatory disease have been reviewed (Grimes and Fry 2015, Weiss et al 2019). Anemia of inflammatory disease may be compounded by other conditions, such as blood loss and nutritional deficiencies.
- Suppression of erythropoiesis by inflammatory cytokines: Decreased RBC production is more likely to occur in chronic inflammatory states because it takes a while for the anemia to develop, due to the long RBC lifespan (i.e. an anemia will develop quicker in a cat due to their shorter RBC lifespan of around 60 days versus dogs, which have a RBC lifespan of 120 days). Cytokines suppress erythropoiesis in multiple ways including:
- Decreased iron availability from decreased absorption (mostly due to cytokine-mediated upregulation of hepcidin, a protein which inhibits iron absorption) and iron sequestration within macrophages (due to increased iron uptake and storage from cytokine-mediated stimulation of ferritin production and hepcidin-mediated decreased iron export in cells; hepcidin causes degradation of ferroportin, which allows iron egress out of cells). This is thought to be one of the main mechanisms of anemia of inflammatory disease. Interleukin-6 is one of the main cytokines responsible for hepcidin upregulation. The effect of hepcidin is compounded by reduced erythropoietin action, because the latter usually upregulates proteins that inhibit hepcidin, e.g. erythroferrone. The mechanisms responsible for reduced red blood cell production in this iron-sequestered state are unclear. However decreased iron inhibits erythropoiesis, by decreasing the number of transferrin receptors on erythroid progenitors. With anemia of inflammatory disease, the increased iron sequestered within macrophages may actually promote synthesis of ferroportin, which may partly offset the hepcidin- and other cytokine-mediated downregulation or degradation, allowing heme synthesis to continue and preventing a severe anemia in contrast to that seen with a true iron deficiency anemia. Although there is, in theory, less iron available for erythropoiesis, the anemia is not typically microcytic or hypochromic. Cats with evidence of iron-deficient erythropoiesis based on low hemoglobin content in reticulocytes were more likely to have evidence of inflammation, but the incidence of this was low in one study of 275 cats (Keiner et al 2020).
- Inhibition of erythroid progenitors by inflammatory cytokines (e.g. tumor necrosis factor [TNF]-α and interferon- γ [IFN-γ]) and hypoferremia.
- Inflammatory cytokines can inhibit the ability of blast-forming unit-erythroid or BFU-E to differentiate or alter the bone marrow environment, affecting erythropoiesis. Inflammation shifts hematopoiesis to myeloid production (via the transcription factor, PU.1) and away from erythropoiesis (by downregulating GATA-1, an erythropoietic transcription factor). Ceramide or free radicles may also cause apoptosis of erythroid progenitors. Inflammatory cytokines can also lead to hematopoietic stem cell exhaustion. IFN-γ also downregulates transferrin receptors on erythroid progenitors.
- Low iron can inhibit erythropoiesis through downregulating transferrin receptor expression on erythroid precursors, decreasing their ability to proliferate (Khalil et al 2018).
- Inhibition of erythropoietin production or release, e.g. IL-1, TNF-α affect production in the kidney by downregulating transcription factors, such as GATA-2. Free radicles can also damage erythropoietin-inducing interstitial cells, leading to decreased production.
- Decreased biologic activity of erythropoietin (erythropoietin-resistance): Inhibition of erythropoietin-mediated proliferation and differentiation, e.g. IL-1 and IL-6 can interfere with signaling via the erythropoietin receptor. Erythropoietin receptor expression may also be downregulated if erythroid cells are iron deficient or inhibited by interferon-γ.
- Decreased RBC lifespan: This has been documented in mice (due to splenic macrophage erythrophagocytosis [Libregts et al 2011]) and cats with inflammation, although the mechanism was not elucidated in cats (Weiss and Kriehbiel 1983). A hemolytic process may also result in a rapid decrease in hematocrit in cats after hospitalization for inflammatory conditions, although other factors (e.g. surgical blood loss, fluid therapy) may have contributed to the anemia or unmasked an already existing anemia (Ottenjan et al 2006). Decreases in RBC count, hemoglobin and hematocrit occur 24 hours after dogs were given 200 ug/kg endotoxin intravenously (with anemia in some of the dogs) and was associated with lower albumin and higher globulin concentrations. This change in RBCs could be due to fluid shifts or a mild hemolytic process (there was no change in total bilirubin concentrations, however liver uptake of unconjugated bilirubin may have kept up with unconjugated bilirubin production from hemolysis) (Higgins et al 2003). Adjuvant-induced inflammation also results in a mild non-regenerative anemia within 7-14 days in dogs, with increased plasma iron turnover, suggesting a hemolytic process (Feldman et al 1981). Inflammatory cytokines (TNFα), complement and oxidant injury from free radicles can decrease RBC lifespan by damaging RBCs and inducing binding of antibodies to RBC surfaces, i.e. the damaged or complement/antibody bound RBCs are prematurely phagocytized by macrophages in the spleen and liver (i.e. extravascular hemolysis). Cytokine-induced activation of macrophages can promote RBC destruction in these sites. The cytokines concurrently suppress the bone marrow response and iron is limited, so the anemia is non-regenerative. Mouse models of anemia of inflammatory disease (and hemolytic anemia) have shown that monocytes transiently convert into erythrophagocytic macrophages in the liver (Theurl et al 2016).
Other clinical pathologic features that may be seen with anemia of inflammatory disease (but also may be absent):
- Hemogram:
- Inflammatory leukogram: Mature neutrophilia with a monocytosis (in dogs, particularly), left shift in neutrophils (presence of bands and more immature neutrophils), toxic change. An inflammatory leukogram is not always present in animals with anemia of inflammatory disease and not all animals with inflammatory leukograms are anemic.
- Thrombocytosis: Reactive response (increased thrombopoiesis due to inflammatory cytokines).
- Chemistry panel:
- Hyperglobulinemia: If electrophoresis was performed, one may see a polyclonal gammopathy (from an acquired immune response) and an increase in α1 (cats) or α2 (other species)-globulins if there is acute or ongoing inflammation (positive acute phase response). Fibrinogen concentrations may also be increased if measured.
- Hypoalbuminemia: Albumin concentrations may be decreased because it is a negative acute phase protein.
- Decreased iron, percentage saturation, total iron binding capacity (TIBC): Iron goes down acutely with inflammatory disease due to inflammatory cytokine induction of hepcidin. Transferrin (indirectly measured via TIBC) is a negative acute phase protein and will decrease (more slowly than iron) with inflammation. If iron is decreased and the TIBC is normal, the percentage saturation of transferrin will be low. If iron and TIBC are both low, the percentage saturation will be normal (see table in interpretation of iron panels).
- Increased acute phase proteins: Haptoglobin (all species), serum amyloid A (horses, dogs), C-reactive protein (dogs), α1-acid glycoprotein (cats, dogs). These can be measured directly and will go up with acute inflammation.
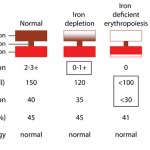
The table below provides useful features (typical findings) that help distinguish between anemia of inflammatory disease and iron deficiency anemia, since both are associated with iron-restricted erythropoiesis (or iron-deficient erythropoiesis in the case of absolute iron deficiency).
| Test | Anemia of inflammatory disease | Iron deficiency anemia |
| Hematocrit | Mildly decreased (e.g. 30-40% in dogs) | Mildly to severely decreased |
| Red blood cell indices | Normocytic normochromic | Microcytic hypochromic or microcytic normochromic |
| Red blood cell morphologic features | No abnormalities* | Hypochromasia (increased central pallor due to decreased rim of hemoglobin) |
| Reticulocyte hemoglobin content (CHretic) or % low CH reticulocytes (% CHretic) | CHretic: ≥ 21.8 pg % CHretic: ≤ 31.3% |
CHretic: ≤ 20.1 pg % CHretic: ≥ 50.7% |
| Iron concentration | Low (usually > 10 ug/dL) | Low (usually < 50 ug/dL) |
| Total iron binding capacity | Normal (acute inflammation) or decreased (longer standing inflammation) | Normal (usually), may be rarely increased or decreased |
| Percentage iron saturation | Low (acute inflammation) or normal (longer standing inflammation), usually >15% | Low, usually <20% |
| * Depends on primary disease (primary disease may be causing RBC abnormalities) | ||
References
Harvey et al 1982. Chronic iron deficiency anemia in dogs. J Am Anim Hosp Assoc 18:946-960.
Chronic kidney disease
Chronic kidney disease can result in an anemia through multiple mechanisms, as indicated below. The anemia is usually non-regenerative and mild to moderate in most animals (95% of dogs in one study [Chervier et al 2012]). Note anemia is not present in all animals with chronic kidney disease and may be seen in some patients with acute kidney injury, where it is likely due to increased hepcidin concentrations (inflammatory disease) with potential concurrent oxidant injury. Echinocytes can be seen in some forms of renal disease (glomerulonephritis).
- Decreased erythropoietin production: Erythropoietin is produced in renal interstitial cells. There is usually not an absolute deficiency of erythropoietin in patients with chronic kidney disease, but erythropoietin levels are not sufficient to sustain erythropoiesis.
- Increased hepcidin: Renal disease is thought to have an inflammatory component, with increased hepcidin causing iron sequestration (functional iron deficiency). The increase in hepcidin can also be secondary to decreased renal excretion (Wojtaszek et al 2020). Inflammatory cytokines can also impair bone marrow responsiveness to erythropoietin. Cats with chronic kidney disease do have biochemical evidence of inflammation, based on serum amyloid A concentrations, and high hepcidin concentrations, which could lead to iron-deficient erythropoiesis (Javard et al 2017).
- Suppression of erythropoiesis: This can occur due to inflammatory cytokines (as indicated above) or “uremic” toxins, both of which can inhibit erythropoiesis or inhibit the action of erythropoietin on erythroid progenitors (some authors refer to this as decreased erythropoietin activity). Uremic toxins can also alter the bone marrow hematopoietic environment, which affects erythropoiesis. This is also one of the main mechanisms for the non-regenerative anemia in chronic kidney disease.
- Decreased RBC lifespan: Uremic toxins can reduce RBC lifespan (promoting extravascular hemolysis). However, due to concurrent suppression or erythropoietin deficiency, the anemia is usually non-regenerative. Oxidative injury to RBCs can also contribute to the anemia in chronic kidney disease.
- Hemorrhage: Animals with uremia frequently suffer from oral and gastrointestinal ulcers which can cause chronic hemorrhage. Iron deficiency may ensue in severely affected animals. However, the anemia is not typically (but can be) regenerative due to the aforementioned reasons.
- Malnutrition: Lack of energy and mineral deficiencies (including iron due to chronic blood loss) will also inhibit erythropoiesis.
Endocrine diseaseEndocrine disorders, such as hypothyroidism and hypoadrenocorticism, can produce a mild to moderate normocytic normochromic anemia (Chervier et al 2012). This is thought to be due to a generalized decrease in metabolism, although there may be an element of anemia of inflammatory disease. These diseases infrequently cause anemia. This anemia is not usually associated with poikilocytosis.Infectious agents
Various types of infectious agents can cause anemia through multiple mechanisms, including extravascular hemolysis (with or without) intravascular hemolysis, and decreased erythropoiesis (direct inhibition of erythropoiesis by the organism or indirect suppression through inflammatory cytokines or anemia of inflammatory disease). Examples are given below (this is not an exhaustive list):
- Erythroparasites: Some species of Mycoplasma (formerly Haemobartonella) spp., Babesia spp, and Anaplasma spp, Cytauxzoon spp, Theileria spp. These parasitize RBC causing premature removal from the circulation (extravascular hemolysis). Secondary immune-mediated removal by macrophages can contribute to the anemia. Some parasites, particularly Babesia and Cytauxzoon also have a component of intravascular hemolysis. Some parasites, e.g .Theileria parva, in cattle and Cytauxzoon in cats, are associated with a non-regenerative anemia (potentially due to infection of erythroid progenitors with Theileria and concurrent anemia of inflammatory disease with Cytauxzoon).
- Trypanosomes: Some species can cause a hemolytic anemia. Trypanosoma cruzi, the species that infects dogs in the USA, causes cardiac disease (Chaga’s disease) and not hemolytic anemia. Similarly, the species infecting cattle (Trypanosoma theileria) in the USA is non-pathogenic (not associated with anemia, at any rate).
- Viruses: e.g. Equine infectious anemia can induce an extravascular hemolysis (attributed to complement fixation on RBC membranes (Sentsui and Kono 1987) as well as a suppression anemia (bone marrow suppression). Feline leukemia virus can induce a non-regenerative anemia due to infection of progenitors in marrow. When the anemia is macrocytic, underlying myelodysplastic syndrome should be suspected in affected cats (myelodysplasia results in the macrocytosis).
- Bacteria: Any type of bacterial infection can cause a non-regenerative anemia due to inflammatory cytokines (anemia of inflammatory disease) if they cause systemic inflammation. Specific bacteria can also induce a hemolytic anemia, including Leptospira and Clostridia (Reef 1983, Weiss and Moritz 2003, Andersen et al 2013). The latter can release toxins which are hemolysins, that can cause intravascular hemolysis, e.g. Clostridium hemolyticum or perfringens, although an immune-mediated component to the anemia can also be seen. Infection with some forms of Escherichia coli (0103:H12), that produce a verocytotoxin, can cause hemolysis as a consequence of hemolytic-uremic syndrome. This is rare in animals and has been reported in a mare with a uterine infection with the causative organism (Dickinson et al 2008) and a dog with hemorrhagic diarrhea (Dell’Orco et al 2003). The hemolysis is this condition is due to a fragmentation anemia from thrombosis in the renal vasculature (extravascular hemolysis), which causes concurrent acute renal failure (likely hypoxic injury) and thrombocytopenia (platelet consumption).
Inherited defectsThese can affect the RBC membrane or metabolic enzyme pathways and should always be considered as a cause of a hemolytic anemia in a young animal. Luckily, they are quite uncommon. Depending on the defect, there may or may not be clues as to the cause of the anemia in a blood smear.
- Membrane defects: Membrane defects decrease RBC lifespan, e.g. stomatocytosis of Alaskan Malamutes, Miniature and Standard Schnauzers and other breeds. Affected animals may not be anemic, but their RBC are macrocytic and hypochromic and have a stomatocytic (mouth-like) appearance in blood smears. A defect in band 3 (anion exchanger) in the RBC membrane is the reported cause of hereditary stomatocytosis in Japanese black cattle. The disorder manifests rapidly after birth and affected animals have a hemolytic anemia with icterus. The mortality rate can be high, with those animals having less severe anemia more likely to survive. Hereditary elliptocytosis has been attributed to a defect in spectrin, a critical component of the RBC cytoskeleton (Terlizzi et al 2009), or band 4.1 (another transmembrane protein) (Smith et al 1983). Affected animals may be asymptomatic. A spectrin deficiency has also been reported in Golden Retrievers, some of which had a hemolytic anemia (Slappendel et al 2005). Although spectrin deficiencies in humans are associated with spherocytosis, spherocytosis were not observed in affected dogs unless they had concurrent IMHA.
- Enzymopathies: These include pyruvate kinase deficiency (e.g. Abyssinian and Somali cats, Beagles, Cairn Terriers, West Highland White Terriers and Basenjis) (Grahn et al 2012, Gultekin et al 2012), phosphofructokinase deficiency (e.g. English Springer Spaniels, American Cocker Spaniels), glucose-6-phosphate dehydrogenase deficiency (American Standardbred) (Stockham et al 1994), and flavin adenine dinucleotide (FAD) deficiency (Mustang) (Harvey et al 2003). Some dogs with pyruvate kinase deficiency have sphero-echinocytes, whereas horses with G6PD or FAD deficiency may have evidence of oxidant injury (eccentrocytes, pyknocytes, Heinz bodies, methemoglobinemia). Phosphofructokinase deficiency can cause an intravascular hemolysis after exercise, due to RBC fragility in an alkaline pH (a respiratory alkalosis is induced by exercise) (Giger and Harvey 1987). For reviews on these disorders, refer to Harvey et al 2006 or Owen and Harvey 2012.
- Nuclear defects: Congenital dyserythropoietic anemia (CDA) has been attributed to defects in nuclear maturation or division in human patients. This has been reported in Polled Hereford cattle with dyskeratosis (Kessell et al 2012) and is likely the cause of macrocytosis in miniature and toy Poodles, although the dogs are not anemic (Canfield and Watson 1989). CDA results in macrocytosis, with marked erythroid dysplasia in the bone marrow.
- Hemoglobin production defect: Inherited defects in hemoglobin production can result in microcytic hypochromic anemia (e.g. thalassemia in humans, which are due to defects in production of the α and β globin chains). These have not been reported in animals (but there are mouse models). Erythropoietic porphyria can result in microcytic RBCs but does not invariably cause anemia (Kuntz et al 2020).
- Unknown defects: A microcytic anemia has been identified in English Springer Spaniels, but the cause is unknown (Holland et al., 1991). This could be due to a defect in hemoglobin production.
Histiocytic disordersDisseminated histiocytic sarcoma (particularly the hemophagocytic variant) and reactive hemophagocytic syndromes may result in an extravascular hemolytic anemia. The anemia may be regenerative or non-regenerative. Spherocytes may be observed in peripheral blood smears, but a Coombs test is usually negative. In this case, the red blood cells are prematurely removed by circulation because of abnormal phagocytic by macrophages, not because of antibody or complement coating of RBC (the macrophage is the problem, not the RBC).DrugsDrugs can cause anemia in multifactorial ways. Any drug is a potential candidate for cause of an idiosyncratic reaction. In some cases, the mechanism of injury is unknown, e.g. DMSO is associated with intravascular hemolysis in some horses via unknown mechanisms (Appell et al 1992).
- Immune-mediated anemia: Some drugs, e.g. penicillin in horses, can cause a secondary happen-mediated hemolytic anemia (Blue et al 1987). The use of recombinant human erythropoietin results in a pure red cell aplasia in dogs, cats and horses secondary to production of anti-erythropoietin antibodies (Randolph et al 1999, Piercy et al 1998, Randolph et al 2004).
- Suppression of erythropoiesis: This could occur through a direct toxic effect of the drug (e.g. chemotherapeutic agents, estrogen) or secondary immune-mediated effects.
Hospital-acquired anemiaThis condition is recognized, particularly in critically ill patients in human medicine, and is also called the “anemia of critical illness”. The condition refers to an anemia that develops or worsens after patients are hospitalized and is multifactorial in origin:
- Hemodilution – this may uncover a pre-existing anemia or may cause an anemia if excessive fluids are given.
- Inflammatory cytokines associated with underlying disease inhibit erythropoiesis (direct inhibition of progenitors, blunting erythropoietin release or by stimulating secretion of hepcidin).
- Blood loss associated with repeated blood sampling or surgery
- Nutritional deficiencies
- Potential oxidant injury, resulting in premature clearance of newly formed RBCs (called neocytolysis) (Astin and Puthutcheary 2014).
A retrospective study in 688 dogs and 163 cats showed a higher incidence of anemia in both dogs and cats after a mean of 4 days of hospitalization. The changes in packed cell volume (PCV) from admission to the last day of testing was worse (greater decrease) in dogs treated surgically, suggesting blood loss or hemodilution during surgery was the greatest contributor to the worsening anemia. This study had several limitations, such as it was unclear if patients with diseases causing ongoing hemorrhage or hemolysis were excluded from analysis and if the changes in PCV over time were done in the same patients. This study showed that patients given blood transfusions were less likely to survive. This could be that these animals had more severe disease or that transfusion is not innocuous and blood products should be given judiciously to anemic patients (Lynch et al 2016).Miscellaneous conditionsSevere hypophosphatemia (generally < 1.0-1.5 mg/dL, e.g. post-parturient hemoglobinuria in cattle, dogs and cats with diabetes mellitus treated with insulin), osmotic lysis (water or hypotonic fluid), DMSO (very high doses [Appell et al 1992), acute liver failure (horses, unknown mechanism [Ramaiah et al 2003]), and envenomation (spiders, snakes [Dickinson et al 1996, Willey and Schaer 2002, Pagano et al 2016], and bees [Noble and Armstrong 1999]) can all result in a hemolytic anemia, mostly due to extravascular hemolysis, although intravascular hemolysis has been reported after mass bee sting in a mare (Lewis and Racklyeft 2014). Severe hypophosophatemia, osmotic lysis, DMSO in horses (Appell et al 1992), equine acute liver failure (possibly due to an emulsifying effects of bile salts [Ramaiah et al 2003]), and snake venoms (Willey and Schaer 2002, Pagano et al 2016) can also result in concurrent intravascular hemolysis.Diagnostic algorithms for anemiaPlease note that these should be used as rough guides. They do not include all scenarios or possibilities, just the more common themes
 |
 |
