Excess fluid can accumulate in body cavities from multiple causes. Characterizing an effusion by its cytologic properties is an important step in diagnosing the cause of the effusion. In dogs and cats, the volume of fluid within the peritoneal, pleural and pericardial space is quite small (generally less than 10ml) and fluid cannot normally be aspirated from these cavities. In contrast, fluid can be collected from the peritoneal cavities in clinically healthy horses, cattle, and camelids and the judgment as to whether there is an abnormal or excessive amount of fluid within the cavity is best made clinically.
Mechanisms of formation
Pleural, peritoneal and pericardial fluid is formed in small amounts in health to allow lubrication and diffusion of substances such as electrolytes. The fluid is formed when plasma exits from a capillary bed in the tissue and enters the interstitial space. The rate of this fluid formation depends, according to Starling’s laws, on the balance between plasma and tissue oncotic and hydrodynamic pressures. The forces promoting fluid filtration out of the capillary on the arteriolar side into the interstitium (plasma hydrodynamic pressure and tissue oncotic pressure) are slightly higher than the forces promoting fluid absorption from the tissue back into the capillary on the venule side (plasma oncotic pressure and tissue hydrodynamic pressure). This leads to a net accumulation of small amounts of fluid in the interstitium that diffuses between the mesothelial cells and enters the body cavity. Excess fluid is normally removed by the lymphatic system.
The main mechanisms by which an effusion can occur are:
- Transudation: This is due to altered hydrodynamic forces within the vessels serving or draining capillary beds or the interstitium (lymphatics).
- Increased plasma hydrodynamic pressure, this is usually from increased venous pressure (e.g. venous hypertension) but can be from increased blood delivery to capillary beds (arterial hypertension or dilation), although the latter is less common.
- Decreased lymphatic drainage (increases tissue hydrostatic pressure)
- Decreased plasma oncotic pressure (hypoalbuminemia)
- Exudation: This is due to increased capillary permeability and is mediated by vasoactive mediators, usually as a consequence of inflammation. This may or may not be accompanied by chemotaxis of leukocytes in response to inflammatory cytokines.
- Vessel or viscus rupture or leakage: Some of these, e.g. bile peritonitis, uroabdomen or gastrointestinal rupture will incite a secondary inflammatory response or exudative effusion but initially may present as a “transudative”-like effusion, with respect to total protein and nucleated cell counts.
- Hemorrhagic effusion: Damage to or rupturing of vessels or vascular organs (e.g. liver or spleen), or a coagulopathy usually affecting secondary hemostasis
- Bile peritonitis
- Uroabdomen
- Gastrointestinal (GI)
Fluid collection and analysis
Collection
If a sufficient volume of fluid is obtained, body cavity fluids should be collected into an EDTA-anticoagulant (purple top) tube. This tube is preferred because cell features are better maintained and bacterial proliferation is inhibited. Concurrent collection of fluid into a non-anticoagulant (red top) tube is always useful with bloody fluids or in case additional testing is desired, e.g. creatinine, total bilirubin. Similarly, fluid can be placed into sterile media for culturing. Smears should be prepared from the fluid to prevent storage-associated artifacts (e.g. phagocytosis of erythrocytes and bacteria by macrophages). At the minimum, a direct smear (from unconcentrated fluid) should be made from the fluid, however if the sample is poorly cellular, there will be insufficient cells to examine. With a poorly cellular sample, it is worthwhile making smears of concentrated fluids (after centrifugation), using only a portion (and not all) of the fluid. If cell counts are not known, cellularity of fluid can be judged somewhat by the turbidity of the fluid. A clear transparent or slightly opaque fluid is likely to be of low cellularity. Direct smears should suffice for a flocculent or non-opaque fluid. For more information on sample collection, refer to related links below.
Fluid analysis
Fluid analysis begins during collection of the sample, with observation for blood contamination, ease of collection, and how much fluid is present in the animal. Then the fluid is analyzed in the laboratory, with the following components being provided (at Cornell University, at any rate):
- Gross evaluation: Color, clarity, and viscosity. Some examples: if the fluid is white and opaque it will most likely be chylous in nature; if the fluid is from a cat and is yellow and highly viscous (perhaps with a fibrin clot], infection with feline infectious peritonitis virus should be considered; red-tinged fluid indicates blood contamination or hemorrhage into the cavity.
-
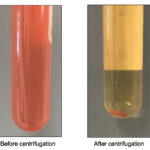
Spun and unspun fluid 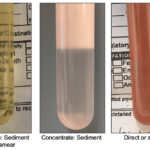
Grossly different fluids Cell counts: Nucleated and RBC counts are performed by automated methods but can be done using manual methods (hemacytometer). Note, nucleated cells include leukocytes and mesothelial or cancer cells. It also can include non-nucleated cells, such as bacteria and intestinal contents (so never rely on counts – always look at a smear).
- Protein content: This is estimated on a supernatant of the fluid (if cloudy or turbid) using a refractometer. A more accurate protein can be obtained on automated chemistry analyzer, however this is more expensive and not typically done. Other laboratories also provide specific gravity instead of, or in conjunction with, a total protein estimate. A study in horses showed that K3EDTA yielded higher total protein by refractometer than non-anticoagulant tubes with differences up to 2.8 g/dL (average of 1.5 g/dL) in 26 peritoneal fluid samples. Spiking samples with K3EDTA resulted in higher protein concentrations via refractometry than a dye-binding Biuret method, with discrepancies being higher with higher EDTA concentrations (Estepa et al 2006). Internal studies at Cornell indicate that the liquid in EDTA tubes by itself has a refractive index of 9 and may result in over-estimation of protein concentrations by refractometry in underfilled samples. Whether this artifact is still present in newer EDTA tubes containing lyophilized EDTA remains to be determined.
-
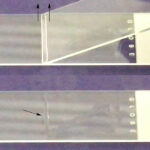
Line smear Cytologic evaluation: This is then performed by microscopic examination of a Wright’s or Diff-Quik-stained smear. The type of smear made will depend on the cellularity of the sample. Fluids with very high nucleated cell counts (>20-30,000/uL) can be prepared without concentration of the cells (direct smear), while concentration techniques such as sediment smears, line (see image on right) or cytospin smears are recommended for fluids with lower cells counts. At Cornell University, we perform direct smears on all fluids, sediment smears with counts between 3 and 30,000/uL, and cytospin smears for fluids with counts <3,000/uL. If fluid volume is insufficient for counts, we gauge cellularity by turbidity or the direct smears that are prepared. If the fluid is very bloody (>1 million RBC/uL), we perform buffy coat smears. The following cells are normally present in cavity fluids, although macrophages and non-degenerate neutrophils dominate (in various proportions).
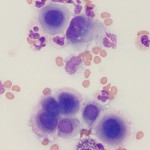
Mesothelial cells 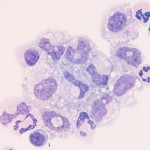
Normal fluid cells - Macrophages: These are present in all types of effusions. They are large cells with irregular cell contours, irregularly shaped nuclei and blue cytoplasm. These can normally demonstrate some degree of leukophagia, contain residual debris of phagocytized material and can have low numbers of cytoplasmic vacuoles.
- Neutrophils: These are non-degenerate.
- Lymphocytes: These are mostly small cells and are present in low numbers.
- Eosinophils and mast cells: Low numbers may be present.
- Mesothelial cells: These are the lining cells of serosal cavities. Spontaneously exfoliated mesothelial cells are round cells with round, central nuclei. Cells may differ in size but nuclei should be nearly uniform in size. The cytoplasm is blue to pink and a pink “halo” can be seen around some cells. In the presence of effusions, the mesothelium may become reactive and proliferate. Under these circumstances clusters of intensely blue mesothelial cells can be seen in the fluid. Some cells may be binucleated and mitotic figures may be observed. Mesothelial cells are not always easily distinguished from macrophages or tumor cells by their morphologic features. Mesothelial cells are not phagocytic but they can take up small particles, e.g., bile crystals and hemoglobin. They may contain stainable iron (hemosiderin) in cases of longstanding hemorrhagic effusion. Mechanically desquamated mesothelial cells (exfoliated by the needle) can be seen and are usually found as flat sheets of uniform cells with a spindled appearance (versus the round appearance of naturally exfoliated cells). It is difficult to differentiate reactive from neoplastic mesothelial cells (i.e. mesothelioma) and reactive or neoplastic mesothelial cells from a carcinoma. Cytologic criteria of malignancy can help differentiate reactive from neoplastic cells, however this does not help with differentiation between a mesothelioma or carcinoma. Fortunately, mesotheliomas are quite rare in contrast to carcinomas, which are far more common.
Cytologic classification
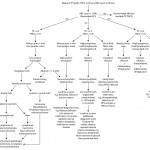
On cytologic examination, we generally classify effusions into one of the above mechanistic categories (to the best of our ability), but add one more category – effusions due to neoplasia. In reality, neoplastic effusions are caused by one (or more) of the above 3 mechanisms. Mechanistic classification of effusions is useful forhelping narrow the list of differential diagnoses in a patient, but often is not diagnostic of a specific disease (i.e. we do not always identify the cause for the effusion in smears). Ultimately, determination of the mechanism and cause for the effusion relies upon a combination of cytology, other clinical pathology and additional diagnostic testing (e.g. imaging results, histopathologic examination of tissues). In the past, effusions were classified on the basis of nucleated cell count, total protein concentration and proportion of cell types into pure transudate, modified transudate, chylous effusion, hemorrhage and exudate, however this classic cytologic approach is less useful and we prefer a mechanistic categorization as shown in the table and diagnostic algorithm.
|
Total protein (g/dL)* |
Nucleated cells (x103/ul)* |
Cytologic findings | Common causes | |
| Transudate: Low-protein, protein-poor or pure)** |
<2.5 |
<1.5 (pure transudate) |
Mostly macrophages, with low numbers of neutrophils, lymphocytes and mesothelial cells; fluid is usually colorless to light yellow, and transparent; may see erythrophagia (hemorrhage) | Pleural fluid: Increased venous or lymphatic hypertension (non-exfoliating neoplasia, lung lobe torsion, etc). Peritoneal fluid: Portal (presinusoidal) hypertension (liver disease), severe hypoalbuminemia (< 1.5 g/dL, an uncommon cause of effusion by itself, often combined with portal hypertension), non-exfoliating neoplasia |
| Transudate: High-protein, protein-rich or modified transudate** |
>2.5 |
<5.0 |
Mostly macrophages and neutrophils, and low numbers of lymphocytes and mesothelial cells (may be in clusters), variable RBC ± erythrophagia (concurrent hemorrhage), plasma cells may be seen (localized antigenic stimulation). Fluid is usually light to moderate yellow, may be blood-tinged and transparent to slightly cloudy (from WBC and RBC) | Pleural fluid: See above and cardiac disease in dogs and cats. In dogs, it is usually due to biventricular failure or left-sided heart failure. With the latter also resulting in right heart failure, pleural effusion can occur with ascites and is less severe than the ascites. In cats, effusions are seen with left and right heart failure and are associated with worse left atrial function. Inflammation affecting pleural vasculature can also result in an effusion with these characteristics. Peritoneal fluid: Sinusoidal or post-sinusoidal hypertension, e.g. cardiac disease, liver disease, non-exfoliating neoplasia |
| Chylous effusion (type of transudate): Chylomicron-rich |
Usually >2.5 (may be inaccurate because large lipoproteins contributes to refractive index) |
Variable, usually >3.0 |
Small lymphocytes often predominate, with low numbers of reactive or large lymphocytes, unless concurrent inflammation (irritating effects of chyle), macrophages and neutrophils may contain numerous discrete margined vacuoles in cytoplasm (lipid). Fluid is grossly white to pink (if concurrent hemorrhage), opaque, and may form a cream layer on standing due to chylomicrons with opaque supernatant. High triglycerides, usually > 100 mg/dL in fluid and usually >2x serum, unless animal is inappetent. | Pleural fluid: Cardiac disease (cats), idiopathic (dogs, cats), neoplasia (dogs and cats, e.g. thymoma) Peritoneal fluid: Rare (lymphangiectasia, abdominal adhesions, mesenteric granuloma/abscess, neoplasia) |
| Lymphocyte-rich effusions |
Variable, depending on cause |
Variable, depending on cause |
Small lymphocytes often predominate, with low numbers of reactive or large lymphocytes. Fluid can be chylous (chylomicron-rich) or non-chylous, resembling a transudative effusion. | Pleural fluid: See above causes for chylous effusions, thymoma, small cell lymphoma (rare) Peritoneal fluid: See above causes; small cell lymphoma (rare) |
| Exudate |
Usually >2.5, often > 4.0 g/dL |
Usually >5.0 |
Neutrophils often predominate; cause may be identified, if acute. With time or certain causes (e.g. fungi, cancer) may see mixed inflammation with mostly neutrophils but low numbers macrophages, and a few lymphocytes (some can be reactive or plasma cells). Hemorrhage can be concurrent (erythrophagia, hematoidin crystals, hemosiderophages). Cells sediment on centrifugation, yielding clear supernatant (unless concurrently chylous). | Multiple, e.g. bacteria, fungi (e.g. Candida), parasites (e.g. Mesocestoides) ruptured/leaking viscus, underlying cancer, sperm (seminoperitoneum from rupture of uterus or vagina during mating), uroperitoneum, bile peritoneum, pancreatitis, etc |
| Feline infectious peritonitis | Usually > 2.5, often > 4.0 g/dL | Usually <5.0 (can be higher) | Variant of an exudative effusion characterized by increased permeability (>2.5 g/dL protein) often unaccompanied by leukocyte chemotaxis (<5.0 TNCC), mixture of mildly degenerate (vacuolated) neutrophils with fewer but increased macrophages, proteinaceous background (crescents, stippled) with fibrin clot; grossly viscous light to moderately yellow fluid with fibrin tags. | |
| Neoplastic effusion |
Variable, frequently >2.5 |
Variable |
Neoplastic cells identified in fluid; may see concurrent inflammation (tumor necrosis, liberation of inflammatory cytokines in response to the tumor or damage-associated molecular patterns from tumor or immune cells). Causes effusion through multiple mechanisms, including hemorrhage (e.g. ruptured splenic hemangiosarcoma), transudative (high- and low-protein) and exudative. May also cause biliary, urinary or GI rupture (uncommon), with secondary sepsis (with GI rupture, e.g. intestinal lymphoma). | Round and epithelial cell tumors exfoliate more readily than other tumors; mesothelioma (rare, can mimic carcinoma and reactive mesothelial cells) Round cell: Lymphoma, mast cell tumor, plasma cell tumor (rare), acute myeloid leukemia (rare) Epithelial cell: Carcinoma (various, e.g. pancreatic, bronchoalveolar, gastric) |
| Hemorrhagic effusion: > 0.5-1 million/uL RBC with a measurable packed cell volume (usually >1%) |
Usually >2.5 |
Depends on peripheral blood count and if there is concurrent inflammation |
Many RBCs, no or very few platelets when acute or longer-standing. Does not clot in a red-top or non-anticoagulant tube. With longer-standing effusions (usually >24 hours), see erythrophages, hemosiderin (in macrophages primarily), and hematoidin crystals (breakdown product of heme ring, i.e. variant of bilirubin crystal) in background and within macrophages. | Pericardial fluid: Pericarditis (idiopathic), hemangiosarcoma All fluids: Trauma, ruptured spleen (e.g. hematoma) or liver (e.g. amyloidosis), neoplasia (e.g. hemangiosarcoma), secondary hemostatic dysfunction (e.g. anticoagulant rodenticide toxicosis) |
| Biliary rupture |
Variable, usually >3.0 |
Variable, usually >5.0 |
Can have low protein and cell count initially and become exudative over time; exudative with yellow-brown to green-brown bile extracellularly and within macrophages (differentiate from hemosiderin with Prussian blue stain); may see “white” bile (mucus – differentiate from fibrin, can also see with mucin-producing neoplasia, e.g. adenocarcinoma); patient may be icteric, confirm with bilirubin measurement (usually > 2x serum, but not always with “white” bile). | Trauma, mucocele (canine), cholelithiasis, neoplasia, or inflammation in biliary tree or gall bladder |
| Uroperitoneum |
Variable |
Variable |
Initially low protein and cell count (dilution with urine, if large rupture in bladder versus small tear in bladder or ureters), becoming exudative with time; confirm with creatinine measurement (usually >2x serum) | Trauma, urolithiasis, neoplasia or inflammation in urinary tract |
| Gastrointestinal rupture | Variable | Variable (often unreliable) | Food material and/or phagocytized bacteria with peritoneal fluid cells (usually neutrophils, but also macrophages), neutrophils are quite degenerate typically. May see concurrent hemorrhage. Must be differentiated from a partial enterocentesis, which contains a mixture of peritoneal fluid cells and possibly phagocytized bacteria if sample is stored before analysis, but neutrophils are not degenerate and patient is not as sick as would be expected from a GI rupture. | Trauma, severe inflammation, obstruction, torsion, intussesception, etc |
|
*Approximate guidelines. |
||||
