Interpretation
Suppurative inflammation with increased eosinophils
Explanation
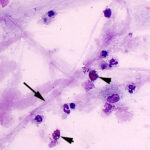
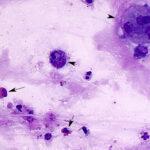
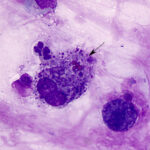
Cytologic examination of sediment smears of the tracheal wash revealed numerous inflammatory cells, which were mostly neutrophils (88%), with fewer macrophages (4%), and eosinophils (8%) (Figures 1-2). Extracellular gram-negative bacterial rods were observed (Figures 1-3) and rare putative degrading organisms were seen intracellularly (not shown). Several multinucleated macrophages were present in the smears and macrophages contained phagocytized neutrophils, eosinophil granules and other debris in their cytoplasm (Figure 2). In the background, there were strands of stringy mucus and a few clusters of columnar epithelial cells, which were bluer than normal (mild atypia attributed to inflammation, not shown). The unusual finding in our experience was the increased percentage of eosinophils and phagocytized eosinophil granules, which indicated underlying eosinophilic tissue inflammation secondary to a parasite, such as the migrating ascarid, Parascaris equorum (Question 1). The bacteria were a single species and, given the neutrophilic inflammation, putative intracellular organisms and imaging findings, were considered to be contributing to or causing the inflammation. Fecal floatation should be the next step to confirm parasitic infection and identify the pathogen (Question 2), considering that culture had already been requested on the sample.
The microcytosis on the hemogram and high red blood cell count were attributed to age-associated changes. The increased total leukocyte count was due to the neutrophil count being close to the upper reference limit. The increased fibrinogen concentration by heat precipitation was mild and, given the crude nature of the test (only changes in 100 mg/dL quantities), not considered to be indicative of inflammation, based on the lack of increase in serum amyloid A concentrations, a more sensitive marker of acute inflammation.
 |
 |
 |
Additional tests
While bacterial culture results were pending, the foal was started on antibiotic therapy for a suspected pneumonia. Culture revealed Nicoletella semolina, an organism of unknown clinical relevance, since it can be present in the airway of normal healthy horses and those with respiratory disease.1 A fecal flotation yielded Eimeria oocysts and Parascarid eggs, confirming a Parascaris infection, so fenbendazole dewormer was administered. After 4 days in the hospital, the foal was doing well and discharged to the care of the owners. The case was then lost to follow-up.
Discussion
Parascaris equorum, part of the Ascaridoidea superfamily, is the largest nematode of horses and one of the most commonly diagnosed parasites in foals and young horses. The life cycle of the roundworm (ascarid) involves migration through the liver and lungs, termed “hepatotracheal migratory route”.2 Infection occurs when parasite eggs are ingested, most often from pasture, which liberates second stage larvae. After passing through the acidic conditions of the stomach, then basic conditions of the small intestine, the eggs lose their protein coating so that larvae can penetrate through the gut wall. The larvae migrate via lymphatics or venules to the liver within 2 to 7 days post-infection. Ascarids are notorious for producing multifocal, small, white, fibrotic hepatic lesions, termed “milk spots”. Two weeks post-infection, migrating third-stage larvae make their way to the lungs via venules, rupturing alveolar membranes. The larvae ultimately migrate into the airway, are coughed up into the pharynx, and swallowed where they can remain in the stomach. Adult roundworms continue to grow within the gut for several months, with mature females reaching up to 23 inches in length. Eggs appear in the feces about 75 to 90 days post-infection. The eggs have a protective, sticky coating that adheres to blades of grass, hairs, or even mares’ udders, which are all potential sources of infection. The eggs last between 1 to 5 years in the environment. Within two weeks after fecal shedding, the eggs become infective and the cycle continues. Sucklings, weanlings, and yearlings are the most commonly affected age groups, but most horses eventually develop an acquired immunity to P. equorum.
Clinical signs and clinicopathological findings in horses affected with P. quorum depend on the animal’s age, parasite location/load, and migration route. Even though infection prevalence with P. equorum is high, morbidity is low.3 Despite the involvement of liver parenchyma in typical P. ascaris infections, clinical signs of liver disease or increased liver enzyme activities have not been associated with infection.4 The main clinical concern with P. equorum infection is small intestinal impaction (colic) due to the growing population of roundworms in the intestine. Surgical treatment of impaction has been documented to have better success if the roundworms were manually milked into the cecum while avoiding opening the small intestine.5 However, ascarid impaction is still a relatively rare scenario.
After experimental inoculation of 2-to-4-week-old foals with approximately 8000 P. equorum eggs, clinical signs referable to the respiratory tract, including coughing and mucoid or purulent nasal discharge, developed 13 to 25 days post infection.6 No other clinical abnormalities were noted and thoracic imaging does not reveal abnormalities. When two yearlings, 8 to 10 months old, were inoculated with the same dose, more severe clinical signs ensued, manifesting as coughing, serous or seromucoid nasal discharge, depression, inappetence, and decreased body condition. Radiographs revealed bronchopneumonia. P. equorum migration through the lungs results in alveolitis, bronchiolitis, and bronchitis.4 In another experimental infection of P. equorum, larval migration resulted in focal necrosis of the liver with eosinophilic granulomas, eosinophilic lymphadenitis, and focal lung parenchyma necrosis with an interstitial pneumonia revealed on necropsy.7
Regarding the foal in this case, the clinical signs – bilateral serous to mucoid nasal discharge, bilaterally enlarged mandibular lymph nodes, a persistent cough, and harsh lung sounds – were indicative of respiratory disease and were confirmed with radiographs. Differential diagnoses for these findings generally default to infectious organisms, specifically viruses (Equine Herpesvirus type-1 and -4, Equine Influenza), bacteria (Streptococcus equi subspecies equi, Rhodococcus equi),8,9 or ascarid migration with potential secondary bacterial infection resulting in a bronchopneumonia, which was the case in this foal. Other inflammatory airway diseases or asthma, pleuropneumonia, or exercise-induced hemorrhage are more common in older horses (2 years).10 However, given the contagious nature of viral and bacterial infections, affected animals may need to be placed in isolation or quarantine and handled with appropriate biosafety measures to minimize spread to other animals in the hospital.
A diagnostic work-up for equine respiratory disease at our hospital usually includes thoracic imaging (radiography, ultrasonography) and cytologic examination of respiratory secretions, either a tracheal wash (TW) or bronchoalveolar lavage (BAL) or both, followed by infectious disease testing as indicated. However, complete imaging of the respiratory tract is difficult to perform in the field, whereas obtaining respiratory secretions is relatively straight-forward and readily accomplished by private practitioners. A TW (via an endoscope or through the trachea) collects secretions in the distal trachea, representative of the peripheral and central airways from all lung segments and is the preferred technique if an infection is suspected. A TW is also the preferred initial diagnostic method for verminous foal pneumonia, because fecal flotation may be negative despite respiratory signs due to the long prepatent period of 10 to 12 weeks. Foals with viral or bacterial infection usually have neutrophilic inflammation and bacteria may be identified extracellularly or phagocytized within neutrophils or macrophages or both in TW samples. Eosinophils are not expected in tracheal washes from foals with bacterial or viral infections, but can sometimes be seen in clinically healthy foals. In a study of clinically health foals aged 1 to 6 months, six out of twenty foals demonstrated greater than 5% eosinophils from tracheobronchial aspirates (upper percentage not stated). When 9 foals were serially tested from 8, 16 and 24 weeks of age, just under 50% of the aspirates had >5% eosinophils (as high as 26% eosinophils). A higher proportion of foals with >5% eosinophils versus <5% eosinophils had detectable mast cells in their tracheal washes (72 versus 21%).11 As in this foal, the high eosinophil percentage was not considered a normal finding but was presumed to be secondary to the lung migratory stage of Parascaris.11 Thus, the presence of eosinophils in TW from foals should raise a suspicion for underlying parasitic infection. P. equorum infection usually manifests on cytologic assessment of tracheal washes with high eosinophil percentages (5-50%) and associated neutrophilic inflammation with a concurrent bacterial infection.12 Our foal had similar findings in the TW. Dictyocaulus arnfieldi is another parasite infecting horse foals, typically when housed with donkeys, but is not commonly associated with clinical disease in foals.6 Other causes of eosinophils in TW samples, such as equine asthma, eosinophilic pneumonia, and multisystemic eosinophilic epitheliotropic disease (see January 2017 Diagnostic challenge) would not be expected in a foal. In contrast to a TW, a BAL uses an endoscope or specialized tubing to retrieve fluid and cells in the distal airways and alveoli and are more representative of interstitial lung disease. A BAL is most often used for non-septic inflammatory conditions, such as equine asthma, which can be associated with an eosinophilia in BAL fluid (i.e. >5% eosinophils).13 Nasopharyngeal swabs are often used for the detection of viral pathogens, usually via testing for viral DNA with polymerase chain reactions.
A fecal floatation and egg count can help confirm a P. equorum infection. Their eggs are yellow-brown in color with a thick, rough outer shell. However, there is no association between fecal egg count and worm load.14 Treatment of verminous pneumonia calls for use of anthelmintics, yet parasitic resistance to ivermectin and pyrantel is becoming increasingly problematic worldwide. For example, in Swedish breeding farms, a high resistance to pyrantel and reduced efficacy of fenbendazole was noted in foals infected with P. univalens in 2018.15 The same group of investigators more recently confirmed fenbendazole resistance to Parascaris species in Swedish horse farms, particularly those with a high foaling rate (>40 foals per year) and higher frequency of antihelmintic treatment.16 This finding raises concern for increasing and more widespread resistance to such commonly used antihelmintics. When treating an infection due to Parascaris species, fenbendazole is the first line of treatment and can still be effective, but fecal reduction tests could be performed 2 weeks after treatment to monitor the efficacy of the dewormer.
Author: Abigail Reid (DVM student, class of 2026); edited by T Stokol.
References
- Hansson, I., Johansson, K. E., Persson, M., & Riihimäki, M. (2013). The clinical significance of Nicoletella semolina in horses with respiratory disorders and a screening of the bacterial flora in the airways of horses. Vet Micro, 162(2-4), 695-699.
- Nielsen, M. K., & Reinemeyer, C. R. (2018). Handbook of equine parasite control (2nd ed., pp. 10-13). Hoboken, NJ: John Wiley & Sons.
- Cribb NC, Cote NM, Bouré LP, Peregrine AS. Acute small intestinal obstruction associated with Parascaris equorum infection in young horses: 25 cases (1985-2004). N Z Vet J. 2006 Dec;54(6):338–43.
- Nicholls JM, Clayton HM, Pirie HM, Duncan JL. A pathological study of the lungs of foals infected experimentally with Parascaris equorum. J Comp Pathol. 1978 Apr;88(2):261–74.
- Tatz AJ, Segev G, Steinman A, Berlin D, Milgram J, Kelmer G. Surgical treatment for acute small intestinal obstruction caused by Parascaris equorum infection in 15 horses (2002-2011). Equine Vet J Suppl. 2012 Dec;(43):111–4.
- Clayton HM, Duncan JL. The migration and development of Parascaris equorum in the horse. Int J Parasitol. 1979 Aug;9(4):285–92.
- Srihakim S, Swerczek TW. Pathologic changes and pathogenesis of Parascaris equorum infection in parasite-free pony foals. Am J Vet Res. 1978 Jul;39(7):1155–60.
- Barr BS. Pneumonia in weanlings. Vet Clin North Am Equine Pract. 2003 Apr;19(1):35–49.
- Punsmann S, Hoppe J, Klopfleisch R, Venner M. Acute interstitial pneumonia in foals: A severe, multifactorial syndrome with lung tissue recovery in surviving foals. Equine Vet J. 2021 Jul;53(4):718–26.
- Ainsworth DM, Hackett RP. Disorders of the Respiratory System. Equine Internal Medicine. 2009 May 18;289.
- Crane SA, Ziemer EL, Sweeney CR. Cytologic and bacteriologic evaluation of tracheobronchial aspirates from clinically normal foals. Am J Vet Res. 1989 Dec;50(12):2042–8.
- Sellon, D.C. &Long, M.T. (2013). Equine Infectious Diseases (2nd ed., pp. 13). St. Louis, MO: Saunders.
- Couëtil LL, Cardwell JM, Gerber V, Lavoie JP, Léguillette R, Richard EA. Inflammatory Airway Disease of Horses–Revised Consensus Statement. J Vet Intern Med. 2016;30(2):503–15.
- Nielsen MK, Baptiste KE, Tolliver SC, Collins SS, Lyons ET. Analysis of multiyear studies in horses in Kentucky to ascertain whether counts of eggs and larvae per gram of feces are reliable indicators of numbers of strongyles and ascarids present. Vet Parasitol. 2010 Nov 24;174(1–2):77–84.
- Martin F, Halvarsson P, Alm YH, Tydén E. Occurrence of fenbendazole resistance in Parascaris spp. on breeding farms in Sweden. Vet Parasitol. 2024 Oct;331:110272.
- Martin F, Höglund J, Bergström TF, Karlsson Lindsjö O, Tydén E. Resistance to pyrantel embonate and efficacy of fenbendazole in Parascaris univalens on Swedish stud farms. Vet Parasitol. 2018 Dec 15;264:69–73.
