Interpretation
Intracytoplasmic morulae in neutrophils, indicating a bacterial infection with an organism such as Anaplasma phagocytophilum
Explanation
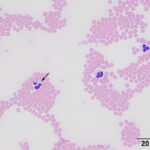
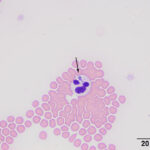
On blood smear review, granular blue inclusions were seen in approximately 7% of neutrophils (Figures 1 and 2) and, rarely, eosinophils (Figure 3), consistent with Anaplasma morulae. The infection would explain the fever and thrombocytopenia (Question 1). Confirmation of the bacterial species causing the infection would require polymerase chain reaction (PCR) testing for bacterial DNA (Question 2). The initial normal anion gap metabolic acidosis was attributed to a primary metabolic acidosis, likely from L-lactate accumulation, given the low normal pH, electrolyte deficits and dehydration. However, L-lactate concentrations were not measured to confirm this interpretation (mild increases in L-lactate concentration do not always increase the anion gap). At first glance, the respiratory alkalosis could be assumed to be secondary to the metabolic acidosis (respiratory compensation), however the pH is normal and not below the reference interval as would be expected for a compensatory response (which does not return the pH to normal), especially given the moderate decrease in bicarbonate and base excess. Use of a correction formula to determine the expected compensation for the degree of acidosis showed an expected pCO2 of 37 ± 3 mmHg (using a factor of 0.7 in the equation) or 34 ± 3 mmHg (using a factor of 1.2 in the equation). This calculated result is higher than the actual result, supporting a primary respiratory alkalosis, likely due to the severe fever, explaining the low normal pH, i.e. there is a mixed primary metabolic acidosis and primary respiratory alkalosis. Respiratory disease, albeit mild, could be contributing to the primary respiratory alkalosis. The increased total solid concentration at intake can be attributed to dehydration and inflammation (increased globulins, given the inflammatory leukogram and rouleaux formation reported in the hemogram and low iron and transferrin saturation on the biochemical panel the next day). The packed cell volume at intake was low normal, but the dehydration may have been masking a mild anemia. In fact, the low normal hematocrit and low red blood cell count and hemoglobin concentration on the hemogram supported a mild anemia, likely due to inflammatory disease. The lymphopenia is attributed to inflammation and stress. The hyperglycemia documented on admission can be explained by stress (endogenous corticosteroids or an epinephrine response or both). The source of the fluid losses, which were ongoing, was not identified (it could be due to extravascular fluid shifts from the fever). The low urea nitrogen and creatinine concentrations can be explained by protein anabolism in a young animal and low muscle mass (the cat was only 6 lb), respectively, combined with overnight fluid therapy. The mild hyperphosphatemia was a physiologic finding attributed to the cat’s age. The trace proteinuria was not considered excessive for the urine specific gravity, which was collected after fluid therapy (precluding an assessment of concentrating ability).
 |
 |
Follow up and outcome
Treatment with doxycycline at a dose of 5 mg/kg per os every 12 hours was initiated. Repeat blood gas analysis showed resolution of the metabolic acidosis, with a normal L-lactate concentration, but an alkalemia due to a primary respiratory alkalosis persisted. The alkalosis resolved two days later. Blood was submitted to the Vector-Borne Disease Diagnostic Laboratory at North Carolina State Veterinary Hospital for a PCR “tick panel”, which detected Anaplasma phagocytophilum DNA. DNA for other agents was not detected (Apicomplexa species, genus of Rickettsia, Babesia, Cytauxzoon, Bartonella, hemotrophic Mycoplasma and Ehrlichia).
The cat was returned for a check-up 3 months after the initial presentation. At this time, the cat was clinically normal and bright and alert. Physical examination was unremarkable. A follow-up hemogram revealed no abnormalities. An explanation for the cat’s mild pulmonary changes on radiographic examination at the initial visit was not found.
Discussion
Anaplasma phagocytophilum is an obligate intracellular rickettsial organism, the causative agent for granulocytic anaplasmosis in several domestic animal species and humans. Anaplasmosis is a tick-borne disease, with the vector tick species varying by geographical location. In the United States, Ixodes scapularis (deer or blacklegged tick) is the main reservoir for this organism in the northeastern and upper midwestern areas, while Ixodes pacificus (western blacklegged tick) is described as the vector in the western part of the country.1
While infections are often described in dogs and horses,2-4 reports in cats are less common. In one PCR survey of 4334 feline blood samples, A. phagocytophilum infection was found in around 1% of cats.5 The low prevalence of infection is hypothesized to be due to the self-grooming habits of cats,1 with most ticks being attached to the dorsal head and neck, an area where cats may have difficulty removing ticks through grooming.6 It has been reported that 24 to 48 hours of tick attachment are required for transmission of A. phagocytophilum.7,8 Cats with outdoor access naturally have an increased risk of vector contact, as seen in the cat of this report, who had outdoor access in a tick-infested wooded area. The cat was from the state of New York, which has endemic tick-borne diseases, including anaplasmosis, in animals and humans.9-11 Several seropositive cases and active infection as demonstrated by PCR have been documented in cats in the northeastern United States.5,6,12
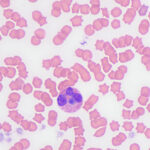
Clinical signs of anaplasmosis in cats are frequently non-specific, as observed in this case. The most common signs include lethargy, pyrexia, and anorexia or hyporexia. Other less common signs, such as ocular discharge, dehydration, tachypnea, abdominal pain, vomiting, and diarrhea, have been reported1,5,13 and several of these signs were seen in this case. A. phagocytophilum infects and replicates in granulocytes. Morulae may be identified in the cytoplasm of neutrophils and less commonly in eosinophils during acute presentation,1,13 facilitating a rapid diagnosis of infection from a blood smear examination, as occurred in this case (Figure 3). One case series reported that 5-20% of neutrophils contained morulae in 11 cats.5 With the exception of intracytoplasmic morulae in neutrophils, clinicopathologic abnormalities are non-specific with A. phagocytophilum infections. Anemia, lymphopenia and thrombocytopenia have been described in low numbers of cases,5 although a direct association with the organism has not been confirmed. Nevertheless, in our and others experience,2 thrombocytopenia is the most common hematologic abnormality in horses with A. phagocytophilum infection and certainly prompts consideration of an infection in this and other species in spring and fall, the peak times of the year for tick activity in upstate New York. Thus, a low platelet count in a febrile cat from an endemic area should prompt consideration of A. phagocytophilum infection, particularly if physical examination or imaging does not uncover a cause for these abnormalities. However, given the tendency of cat platelets to form clumps, thrombocytopenia in automated hematology reports from cats should be viewed with caution until a blood smear examination has been performed. In this case, even though we identified platelet clumps on blood smear examination, we still judged the platelet count as low on a smear estimate, thus confirming the thrombocytopenia provided by our automated analyzer results. Although this cat had an inflammatory leukogram, which we attributed to the bacterial infection, inflammatory leukograms are not always present in horses (the most common species with A. phagocytophilum infections at our institution).
To confirm an A. phagocytophilum infection, additional testing such as PCR and serologic tests can be performed, with the former being the most effective method of showing an active infection. Paired serological testing done 4 weeks apart can provide a diagnosis, although such testing is less practical with delayed results versus PCR analysis. Doxycycline is the drug of choice for clinical anaplasmosis, with clinical responses occurring in most treated cats.5,14
This case illustrates that A. phagocytophilum infection should be considered as a differential diagnosis in cats that are presented for nonspecific clinical signs such as lethargy, anorexia, and fever, especially in cats with outdoor access in endemic areas. Blood smear examination is a useful tool to identify morulae in cases of acute disease, but the absence of morulae does not rule out an infection. In one case series, morulae were only identified in blood smears in around 33% of 11 cases.5 If there is clinical suspicion of this disease, further testing like PCR should be pursued when possible.
Author: I Alvarado-Hidalgo (resident); edited by T Stokol.
References
- Schäfer I, Kohn B. Anaplasma phagocytophilum infection in cats: A literature review to raise clinical awareness. J Feline Med Surg. 2020;22(5):428-441. doi:10.1177/1098612X20917600
- Oliver A, Conrado FO, Nolen-Walston R. Equine Granulocytic Anaplasmosis. Vet Clin North Am Equine Pract. 2023;39(1):133-145. doi:10.1016/j.cveq.2022.11.011
- Thompson D, Thirumalapura NR, Tewari D. Prevalence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in horses from Pennsylvania (2017–2019) using antibody and organism-based detection. J Am Vet Med Assoc. 2022;260(14):1834-1838. doi:10.2460/javma.22.06.0232
- Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59(2):112-120. doi:10.1111/jsap.12787
- Savidge C, Ewing P, Andrews J, Aucoin D, Lappin MR, Moroff S. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J Feline Med Surg. 2016;18(2):85-91. doi:10.1177/1098612X15571148
- Little SE, Barrett AW, Nagamori Y, et al. Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet Parasitol. 2018;257:15-20. doi:10.1016/j.vetpar.2018.05.002
- Hodzic E, Fish D, Maretzki CM, De Silva AM, Feng S, Barthold SW. Acquisition and Transmission of the Agent of Human Granulocytic Ehrlichiosis by Ixodes scapularis Ticks. J Clin Microbiol. 1998;36(12):3574-3578. doi:10.1128/JCM.36.12.3574-3578.1998
- Katavolos P, Armstrong PM, Dawson JE, Telford Iii SR. Duration of Tick Attachment Required for Transmission of Granulocytic Ehrlichiosis. J Infect Dis. 1998;177(5):1422-1425. doi:10.1086/517829
- Russell A, Prusinski M, Sommer J, et al. Epidemiology and Spatial Emergence of Anaplasmosis, New York, USA, 2010‒2018. Emerg Infect Dis. 2021;27(8):2154-2162. doi:10.3201/eid2708.210133
- Bowman D, Little SE, Lorentzen L, Shields J, Sullivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: Results of a national clinic-based serologic survey. Vet Parasitol. 2009;160(1-2):138-148. doi:10.1016/j.vetpar.2008.10.093
- Liu Y, Watson SC, Gettings JR, et al. A Bayesian spatio-temporal model for forecasting Anaplasma species seroprevalence in domestic dogs within the contiguous United States. Dumler JS, ed. PLOS ONE. 2017;12(7):e0182028. doi:10.1371/journal.pone.0182028
- Magnarelli LA, Bushmich SL, IJdo JW, Fikrig E. Seroprevalence of antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am J Vet Res. 2005;66(11):1895-1899. doi:10.2460/ajvr.2005.66.1895
- Graham M, Ewing P, Whelan M. Acute Anaplasma phagocytophilum infection in a pediatric domestic cat. J Feline Med Surg Open Rep. 2023;9(2):20551169231213505. doi:10.1177/20551169231213505
- Pennisi MG, Hofmann-Lehmann R, Radford AD, et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg. 2017;19(5):542-548. doi:10.1177/1098612X17706462
