Interpretation
Myeloid hyperplasia with dysplasia in neutrophils; megakaryocytic hyperplasia with dysplasia, and erythroid hyperplasia with very mild dysplasia.
Explanation
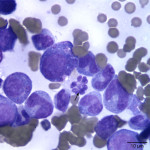
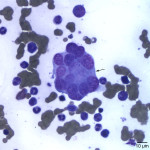
Besides displaying toxic change (compatible with the described toxic features in blood), numerous hypersegmented botryoid neutrophils (Question 1, Figure 2A) and a few giant mature neutrophils were observed in the marrow. Megakaryocytes were mildly dysplastic; there were larger cells with higher nuclear to cytoplasmic ratios than normal, nuclear to cytoplasmic asynchrony characterized by hyperlobulated nuclei in an immature deep blue cytoplasm, (Question 1, Figure 3A), and larger than normal immature megakaryocytes. Rare megaloblasts (dysplastic nucleated erythroid cells) were seen on scanning. The normal peripheral white blood count with a left shift and toxic change indicates an inflammatory leukogram, with a likely peripheral source of inflammation. Inflammatory cytokines would stimulate myelopoiesis and explain the myeloid hyperplasia. The normal white blood count with the myeloid hyperplasia could reflect a balance between production and consumption. However, it is possible that these cells are dying prematurely in the bone marrow (due to the dysplasia in neutrophils). The peripheral thrombocytopenia could be due to a combination of decreased production (supported by the dysplasia in these cells in marrow) and peripheral causes, such as consumption (particularly with the evidence of inflammation), although other mechanisms cannot be ruled out. The marked anemia was also attributed to decreased production (likely due to ineffective erythropoiesis with concurrent suppression from inflammatory disease). All these changes can be attributed to FIV infection (Question 2).
 |
 |
Case follow-up
A bone marrow core biopsy was submitted for histologic evaluation concurrently with the aspirate. Examination of hematoxylin & eosin-stained sections of the core revealed a hypercellular marrow with myeloid hyperplasia and erythroid hypoplasia. There was no evidence of myelophthisis, neoplastic infiltrate or myelofibrosis in the examined sections. Sections of stomach, duodenum, and a mass in the stomach were also evaluated. The sections of stomach showed mild nonspecific gastritis (mild diffuse lymphoplasmacytic, neutrophilic, and eosinophilic infiltrate), consistent with inflammatory bowel disease. The mass was an inflammatory hyperplastic polyp. In the sections of duodenum, there was a mucosal infiltrate of mixed mononuclear cells, predominantly small lymphocytes. Some of the lymphocytes were intraepithelial and they were mostly individual and scattered (they did not form nests or plaques). These findings were considered compatible with chronic inflammatory bowel disease or an early low-grade lymphoma. Additional testing, such as PCR for antigen receptor rearrangement (PARR) or immunohistochemistry was suggested to differentiate between these two possibilities. Despite symptomatic treatment with antibiotics, anti-emetics and anti-diarrheal drugs, the cat showed no improvement and was euthanized, thus no additional testing was performed. It is possible that the gastrointestinal system was the source of inflammation evident in the cat’s leukogram (toxic change).
Discussion
Feline immunodeficiency virus is a lymphotropic retrovirus associated with an acquired immunodeficiency syndrome (AIDS) in cats1,2 that is biologically and virologically similar to human immunodeficiency virus (HIV).3 Transmission occurs primarily by biting and fighting, hence the disease has a higher incidence in free-roaming male adult cats. One to 2 months after infection an acute stage of illness, characterized by fever, diarrhea, depression, and generalized lymphadenopathy occurs.3-5 Clinical signs usually resolve by week 20 after infection and after a long asymptomatic period of chronic infection(months to years), cats can progress to a late symptomatic stage.3 Clinical manifestations of the latter stage consist of generalized lymphadenopathy, diarrhea, anorexia, fever, weight loss, opportunistic infections, neurological and behavioral abnormalities, and neoplasia.6-9 These clinical disorders are associated with a progressive depletion of CD4+ T-lymphocytes, functional T-cell defects and hematologic abnormalities.10-13
Abnormalities in peripheral blood and bone marrow are found in many (71-75%) of FIV-infected cats.14-17 These abnormalities mostly occur in the acute and chronic symptomatic stages of infection, though they have also been reported in asymptomatic cats.4,18-21 They consist of multiple peripheral blood cytopenias accompanied by marrow morphological abnormalities (myelodysplasia), which has been referred as FIV-myelopathy. Another virus commonly associated with myelodysplasia is FeLV. However, unlike FIV-mielopathy, FeLV-induced myelodysplasia (FeLV-MDS) is considered a clonal malignancy that commonly progresses to acute myeloid leukemia (most frequently acute erythroid leukemia).18
A high percentage (20-40%) of FIV-infected cats develop anemia and neutropenia.16 Neutropenia and thrombocytopenia are noted during the acute symptomatic stage of infection,22-25 whereas decreased peripheral blood counts and marrow myelodysplastic changes are more frequent in the symptomatic chronic stage.26-29 Bone marrow abnormalities are characterized by myeloid and erythroid hyperplasia, and reactive lymphocytosis and plasmacytosis. Rarely infiltration with neoplastic lymphocytes or myeloid cells is observed.16 Morphologic abnormalities are similar to those see in HIV-infected individuals30 and include giant band and hypersegmented neutrophils, neutrophils with ring-shaped nuclei, micromegakaryocytes, and megakaryocytes displaying asynchronous maturation and multinucleation (separate nuclei). Abnormal erythroid precursors are less frequent, they include multiple nuclei in erythroid precursors and exceptionally large nucleated red blood cells (megaloblasts), similar to that seen in this case.16,18,30 Usually neutropenia is first noted 6-8 weeks after infection and nadir counts (range, 0.2-2.0 x 103/μL) correlate with appearance of the first clinical signs.3,31 Neutrophilia has been described in some cats with concurrent bacterial infections.9,14,17 Anemia and thrombocytopenia are more common in cats with severe clinical disease.
The asymptomatic stage is not usually associated with peripheral blood cytopenias.16,18 During this stage, the bone marrow is characterized by increased numbers of lymphocytes, plasma cells or eosinophils. The myeloid, erythroid and megakaryocytic lineages show normal maturation.16 However, a recent study detected multiple hematological abnormalities in many (24 of 50 cats) asymptomatic FIV-infected cats. These included anemia, thrombocytopenia, and neutropenia. Multiple cytopenias were more frequent and low numbers (8%) of cats were pancytopenic. Additionally, myelodysplastic changes in the myeloid and megakaryocytic lineages, similar to that seen in this case, were observed.18
The exact mechanism leading to the FIV-associated hematologic abnormalities remains unclear, but multiple potential mechanisms have been proposed. Data from previous studies suggest that hematopoietic precursors are not major targets of FIV.32 Nowadays, the most accepted mechanism is that FIV-induced marrow suppression and dysregulation could be due to virus’s capacity to alter the hematopoietic regulatory function of bone marrow stromal cells.33-35 Bone marrow stromal cells include fibroblasts, endothelial cells, epithelial cells, and resident macrophages. They provide growth factors, cellular interactions, and structural matrix necessary for proliferation and differentiation of hematopoietic stem cells. It is thought that FIV-infected stromal cells, particularly macrophages and fibroblasts, are unable to provide growth factors to support normal hematopoiesis. 35-38 However, the mechanism by which the virus affects the expression or release of growth factors is unclear. Recent reports suggest that either loss of growth stimulators or production of growth inhibitors (e.g., TNF-α) by the infected stromal cells may be the major mechanism involved in altered hematopoiesis.35
The cat in this case apparently presented during the chronic symptomatic stage of FIV infection. The hematologic abnormalities in this case correlate to those commonly associated with FIV. The cat presented with severe non-regenerative anemia and severe thrombocytopenia, both of which were associated with dysplasia (more prominent in megakaryocytes). Myeloid dysplasia was prominent despite a normal neutrophil count in peripheral blood. This could represent concurrent abnormal production of these cells (ineffective myelopoiesis). However, it should be noted that the dysplastic features in predominantly neutrophils and megakaryocytes in marrow can be seen in cats with FIV infection without cytopenias, so other causes for the cytopenias in this cat (e.g. peripheral consumption, decreased production due to inflammatory cytokines that are inhibitory for hematopoiesis, or stromal cell defects) are likely operative in this cat. The increased myeloid precursors in the splenic aspirate were attributed to inflammatory cytokines (IL-1, G-CSF, etc) . The mild hepatic lipidosis was considered secondary to the prolonged anorexia. Since chronic infection with FIV is associated with an increased risk of developing lymphoma, it is possible that the virus may have also been responsible for the suspected low-grade intestinal lymphoma.
References
- Pedersen NC, Ho EW, Brown ML, et al. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987;235:790-793.
- Pedersen NC, Yamamoto JK, Ishida T, et al. Feline immunodeficiency virus infection. Vet Immunol Immunopathol 1989;21:111-129.
- Linenberger ML, Abkowitz JL. Haematological disorders associated with feline retrovirus infections. Baillieres Clin Haematol 1995;8:73-112.
- Yamamoto JK, Sparger E, Ho EW, et al. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res 1988;49:1246-1258.
- Tindall B, Barker S, Donovan B, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med 1988;148:945-949.
- Ishida T, Taniguchi A, Matsumura S, et al. Long-term clinical observations on feline immunodeficiency virus infected asymptomatic carriers. Vet Immunol Immunopathol 1992;35:15-22.
- Pedersen NC, Barlough JE. Clinical overview of feline immunodeficiency virus. J Am Vet Med Assoc 1991;199:1298-1305.
- Sparger EE, Luciw PA, Elder JH, et al. Feline immunodeficiency virus is a lentivirus associated with an AIDS-like disease in cats. AIDS 1989;3 Suppl 1:S43-49.
- Yamamoto JK, Hansen H, Ho EW, et al. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc 1989;194:213-220.
- Ackley CD, Yamamoto JK, Levy N, et al. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol 1990;64:5652-5655.
- Barlough JE, Ackley CD, George JW, et al. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquir Immune Defic Syndr 1991;4:219-227.
- Hoffmann-Fezer G, Thum J, Ackley C, et al. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J Virol 1992;66:1484-1488.
- Novotney C, English RV, Housman J, et al. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS 1990;4:1213-1218.
- Fleming EJ, McCaw DL, Smith JA, et al. Clinical, hematologic, and survival data from cats infected with feline immunodeficiency virus: 42 cases (1983-1988). J Am Vet Med Assoc 1991;199:913-916.
- Hopper CD, Sparkes AH, Gruffydd-Jones TJ, et al. Clinical and laboratory findings in cats infected with feline immunodeficiency virus. Vet Rec 1989;125:341-346.
- Shelton GH, Linenberger ML, Grant CK, et al. Hematologic manifestations of feline immunodeficiency virus infection. Blood 1990;76:1104-1109.
- Sparkes AH, Hopper CD, Millard WG, et al. Feline immunodeficiency virus infection. Clinicopathologic findings in 90 naturally occurring cases. J Vet Intern Med 1993;7:85-90.
- Fujino Y, Horiuchi H, Mizukoshi F, et al. Prevalence of hematological abnormalities and detection of infected bone marrow cells in asymptomatic cats with feline immunodeficiency virus infection. Vet Microbiol 2009;136:217-225.
- Callanan JJ, Thompson H, Toth SR, et al. Clinical and pathological findings in feline immunodeficiency virus experimental infection. Vet Immunol Immunopathol 1992;35:3-13.
- George JW, Pedersen NC, Higgins J. The effect of age on the course of experimental feline immunodeficiency virus infection in cats. AIDS Res Hum Retroviruses 1993;9:897-905.
- Moraillon A, Barre-Sinoussi F, Parodi A, et al. In vitro properties and experimental pathogenic effect of three strains of feline immunodeficiency viruses (FIV) isolated from cats with terminal disease. Vet Microbiol 1992;31:41-54.
- Clark SJ, Saag MS, Decker WD, et al. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med 1991;324:954-960.
- Daar ES, Moudgil T, Meyer RD, et al. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med 1991;324:961-964.
- Gaines H, von Sydow M, Pehrson PO, et al. Clinical picture of primary HIV infection presenting as a glandular-fever-like illness. BMJ 1988;297:1363-1368.
- Kinloch-de Loes S, de Saussure P, Saurat JH, et al. Symptomatic primary infection due to human immunodeficiency virus type 1: review of 31 cases. Clin Infect Dis 1993;17:59-65.
- Castella A, Croxson TS, Mildvan D, et al. The bone marrow in AIDS. A histologic, hematologic, and microbiologic study. Am J Clin Pathol 1985;84:425-432.
- Harris CE, Biggs JC, Concannon AJ, et al. Peripheral blood and bone marrow findings in patients with acquired immune deficiency syndrome. Pathology 1990;22:206-211.
- Spivak JL, Bender BS, Quinn TC. Hematologic abnormalities in the acquired immune deficiency syndrome. Am J Med 1984;77:224-228.
- Zon LI, Arkin C, Groopman JE. Haematologic manifestations of the human immune deficiency virus (HIV). Br J Haematol 1987;66:251-256.
- Ryu T, Ikeda M, Okazaki Y, et al. Myelodysplasia associated with acquired immunodeficiency syndrome. Intern Med 2001;40:795-801.
- Linenberger ML, Beebe AM, Pedersen NC, et al. Marrow accessory cell infection and alterations in hematopoiesis accompany severe neutropenia during experimental acute infection with feline immunodeficiency virus. Blood 1995;85:941-951.
- Linenberger ML, Shelton GH, Persik MT, et al. Hematopoiesis in asymptomatic cats infected with feline immunodeficiency virus. Blood 1991;78:1963-1968.
- Kulkosky J, Bouhamdan M, Geist A, et al. Pathogenesis of HIV-1 infection within bone marrow cells. Leuk Lymphoma 2000;37:497-515.
- Moses A, Nelson J, Bagby GC, Jr. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood 1998;91:1479-1495.
- Tanabe T, Yamamoto JK. Phenotypic and functional characteristics of FIV infection in the bone marrow stroma. Virology 2001;282:113-122.
- Bahner I, Kearns K, Coutinho S, et al. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1-induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood 1997;90:1787-1798.
- Scadden DT, Zeira M, Woon A, et al. Human immunodeficiency virus infection of human bone marrow stromal fibroblasts. Blood 1990;76:317-322.
- Schwartz GN, Kessler SW, Rothwell SW, et al. Inhibitory effects of HIV-1-infected stromal cell layers on the production of myeloid progenitor cells in human long-term bone marrow cultures. Exp Hematol 1994;22:1288-1296.
Authored by: D Hernandez (clinical pathology resident), T Stokol
The authors thank Dr. Marnin Forman for his assistance in providing follow-up information on this case.
