Interpretation
Mixed inflammation with lungworm infection (suspect Muellerius capillaris)
Explanation
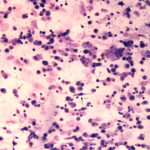
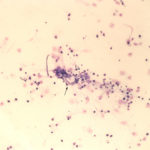
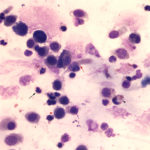
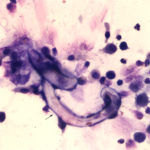
The sediment smears of the fluid from goat #1 contained fat, some mucus, many individualized columnar epithelial cells from the respiratory mucosa and mixed inflammatory cells including neutrophils, eosinophils and macrophages, some of which were binucleated (Figure 1a, Question 1). Macrophages had phagocytized eosinophil granules in their cytoplasm. Rare lungworm larvae were seen on scanning (Question 2). The sediment smears from goat #2 contained chunks of columnar epithelial cells, with low numbers of a mixed inflammatory population as seen in goat #1 (Figure 3a and 4). A few macrophages were binucleated. Numerous lungworm larvae were seen on scanning (Figure 2a, 4). The cytologic interpretation for both goats was mixed inflammation with lungworm larvae. The sediment smears from goat #3 contained large amounts of blood (compatible with the grossly bloody appearance), clusters of ciliated columnar epithelial cells, plant material and protozoa with squamous epithelial cells containing adherent bacteria, with low numbers of mixed inflammatory cells as seen in the other two goats (not shown). A few macrophages were multinucleated. Neither lungworm larvae nor phagocytized bacteria within inflammatory cells were seen. The cytologic diagnosis in the latter goat was mixed inflammation with oropharyngeal contamination (plant material and protozoa were considered compatible with cud).
 |
 |
 |
 |
Additional Diagnostic Testing
The veterinary parasitologist, Dr. Lejeune, in the Animal Health Diagnostic Center was consulted as to the species of the lungworm. He measured five larvae, which ranged in length from 244-270 μm, which is in the range expected for Muellerius capillaris (230-320 μm). A kinked tail was also appreciated in some of the larvae.
Aerobic bacteria and mycoplasma culture was negative and a competitive ELISA for small ruminant lentivirus was negative (0%) in all three goats.
Discussion
Infectious agents, including viruses, bacteria and nematode parasites, are the most common cause of respiratory disease in goats. Viruses such as caprine arthritis encephalitis virus (a lentivirus), Jaagsiekte (a retrovirus), bacteria such as Mycoplasma species, Corynebacterium pseudotuberculosis (caseous lymphadenitis) and Mycobacterium species, and lungworm can cause chronic respiratory disease in goats. In contrast, viral infection due to the paramyxovirus, pestis de petits ruminant, and bacterial infections due to Mannheimia haemolytica or Pasteurella multocida are usually acute in nature, with the latter often exacerbating viral or parasitic diseases. High mortality rates are associated with infections by Mycoplasma capricolum subsp capripneumonia (contagious caprine pleuropneumonia) and Mycoplasma mycoides subsp capri or mycoides and pestis de petits ruminants.1 In contrast, pneumonia secondary to lungworm (verminous pneumonia) can be insidious and subclinical, resulting in ill-thrift and decreased production in milking herds without obvious clinical signs of respiratory disease.1,2
There are several different lungworms that affect goats, including Dictyocaulus filaria and the protostrongyles, Protostrongylus rufescens, Muellerius capillaris, Cystocaulus ocreatus, Neostrongylus linearis and Varestrongylus pneumonicus. The types of protostrongyles found in goats varies by geographic region, but Muellerius and Protostrongylus are seen worldwide, with Muellerius being of much higher prevalence, infecting domestic and wild goats.3-5 Prevalence rates of over 90% have been reported in some herds.6 Dictyocaulus filaria, which resides in the bronchi, is considered the most pathogenic, with more severe infection in kids. Protostrongylus rufescens similarly lives in the bronchi, with clinical disease also in kids. In contrast, Muellerius capillaris resides in the alveoli and produces variably sized pulmonary nodules, typically in the caudodorsal regions of the lung. The infection is often subclinical, but clinical signs of dyspnea and coughing can occur with severe infestations or when there are superimposed bacterial infections and are more severe in adult goats versus kids.1 In one experimental study, kids given infected snails developed a severe cough and dyspnea 3 weeks after infection, followed by abatement of clinical signs with random reoccurrence. The percentage of eosinophils increased in peripheral blood from 0 to a peak of around 7% 4 weeks after infection.7 Such a mild increase may be readily missed on hemograms.
Lungworm infection is typically diagnosed by identification of larvae in feces, using flotation techniques such as Bauerman or McMaster’s.1,2 As shown in these goats, the diagnosis can also be facilitated by cytologic analysis of tracheal wash samples, because protostrongyle larvae or Dictyocaulus larvae and embryonated eggs can be observed in these specimens. The different species can be distinguished by the length of the first stage larvae (230-320 μm for Muellerius, 370-400 μm for Protostrongylus and >500 for Dictyocaulus) and the shape of the tail, which has a dorsal spur in Muellerius and is pointed or blunt in Protostrongylus and Dictyocaulus, respectively.1 Even if no organisms are seen in the tracheal wash fluid (such as the case in one of the goats), the presence of eosinophils within a tracheal wash in a small ruminant should prioritize parasitic infection (from lungworm or a tissue migrating phase of an intestinal parasite), even when seen in low numbers as in these goats. In such cases, assessment of feces for parasitic ova or larvae would be prudent.
There is limited data on expected cytologic findings in tracheal wash samples of goats with verminous pneumonia. In one study of experimental infection of kids with infected snails, bronchoalveolar fluid nucleated cell counts increased within 2 weeks of infection and peaked at around an average of 500 cells/μL (from <50 cells/μL) in kids given a single dose. Nucleated cell counts were higher (average of around 1200 cells/μL) in kids given 2 doses 21 days apart. Mononuclear cells (macrophages and lymphocytes) dominated in the fluid samples, with fewer eosinophils (average, 10-23%, with higher averages in the 2 dose group) and neutrophils (5-10% average). In both groups (n=4 per group), the counts decreased but remained high up to 23 weeks after infection. On histopathologic examination of lung tissue, there was mixed perivascular and interstitial inflammation with type II pneumocyte hyperplasia in kids given one dose. Despite fewer nodules and lower numbers of adult worms, the inflammation was more severe in kids given 2 doses of infected snails with development of eosinophil-rich granulomas, that also contained lymphocytes and neutrophils, and type II pneumocyte hyperplasia. Neutrophils were particularly prominent in lesions with degrading parasites and lesions showing resolution (features not specifically stated).8
The lifecycle of Muellerius capillaris is indirect, with the goat being the definitive host. Intermediate hosts are slugs or snails and goats acquire infection by eating the intermediate host containing the infective third stage larvae or the larvae which are liberated onto the pasture. Once ingested, the larvae penetrate the intestinal wall and migrate to the lungs, with the prepatent period ranging from 4-7 weeks. Once within the lung, the worms reside mostly in subpleural alveoli and incite an inflammatory response, consisting of macrophages, lymphocytes, eosinophils and neutrophils. The inflammation results in diffuse lung disease (called lobular lesions) or distinct nodules, with the latter typically found in the diaphragmatic lobes. More acute nodular lesions are hemorrhagic, whereas older lesions become granulomatous and mineralized and may contain degrading or fewer adult worms.6-10 In one study, diffuse lesions consisted of type II pneumocyte hyperplasia, large numbers of worms, eggs and larvae, and neutrophilic, eosinophilic and plasma cell infiltrates, with regional lymphoid hyperplasia. Other diffuse lesions contained fewer viable worms and less eggs and larvae.7 Another study found that diffuse lesions contained more Protostrongylus refuscens and Cystocaulus ocreatus than Muellerius capillaris, but this may be a geographical finding.6 Immunity to the parasite is poor, with increasing severity of inflammation with reinfection, despite decreased numbers of adult worms in the lungs.8
Treatment of infected goats is difficult because most antihelmintics do not eliminate infection and only decrease larval shedding temporarily.11 A 2016 study showed that moxidectin was more effective in eliminating larval shedding, with decreased shedding observed for up to 70 days after a single treatment with 1 mg/kg dose in a herd of 10 goats. In one of the 10 treated goats, shedding resumed by day 77 with similar numbers of larvae shed by day 98. The latter goat was euthanized at the end of the study and still had inflammatory infiltrates with viable eggs and larvae. In a second euthanized goat which had a complete reduction in fecal larval counts, thickened alveolar septae were seen, along with inflammatory cells, including granulomas. Viable or degraded parasites were not described.12 Elimination of the intermediate host is ideal, but this is virtually impossible in animals kept on pasture and herds may just have to “live” with the infection, particularly if drug resistance to moxidectin emerges (a matter of time) or is shown in more extensive studies.
References
- Smith MC, Sherman DM. Respiratory System in Goat Medicine, 2nd ed; Smith MC, Sherman DM eds. Wiley-Blackwell, Ames, Iowa; pp 339-377.
- Panuska C. Lungworms of ruminants. Vet Clin North Am Food Anim Pract 2006;22:583–593.
- Domke AVM, Chartier C, Gjerde B, et al. Prevalence of gastrointestinal helminths, lungworms and liver fluke in sheep and goats in Norway. Vet Parasitol 2013;194:40–48.
- Cassini R, Párraga MA, Signorini M, et al. Lungworms in Alpine ibex (Capra ibex) in the eastern Alps, Italy: An ecological approach. Vet Parasitol 2015;214:132–138.
- Berrag B, Cabaret J. Assessment of the severity of natural infections of kids and adult goats by small lungworms (Protostrongylidae, Nematoda) using macroscopic lesion scores. Vet Res 1997;28:143–148.
- Ashraf M and Nepote KH. Prevalence of gastrointestinal nematodes, coccidian and lungworm in Maryland Dairy Goats. Small Rumin Res 1990;3:291-298.
- Sauerländer R. Experimental infection of sheep and goats with Muellerius capillaris (Protostrongylidae, Nematoda). Zentralblatt Vet Reihe B J Vet Med Ser B 1988;35:525–548
- Berrag B, Rhalem A, Sahibi H, et al. Bronchoalveolar cellular responses of goats following infection with Muellerius capillaris (Protostrongylidea, Nematoda). Vet Immunol Immunopathol 1997;58:77-88.
- Berrag B, Bouljihad M, Cabaret J. A quantitative approach to nematode lung worm burdens in goats. Parasite Paris Fr 1996;3:291-295.
- Panayotova-Pencheva MS, Alexandrov MT. Some pathological features of lungs from domestic and wild ruminants with single and mixed protostrongylid infections. Vet Med Int 2010;2010:741062.
- Helle O. The efficacy of fenbendazole and albendazole against the lungworm Muellerius capillaris in goats. Vet Parasitol 1986;22:293–301.
- Vadlejch J, Makovický P, Čadková Z, et al. Efficacy and persistent activity of moxidectin against natural Muellerius capillaris infection in goats and pathological consequences of muelleriosis. Vet Parasitol 2016;218:98–101.
Authored by: T Stokol.
