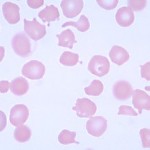
The term “poikilocyte” is a generic or umbrella term to describe erythrocytes with abnormal shape. We can subclassify poikilocytes by specific shape changes, some of which have fairly unique diagnostic significance, while other forms are quite non-specific. Wherever possible, the red blood cell shape should be identified specifically, e.g. acanthocyte, keratocytes, echinocytes, and the generic term of poikilocytosis or poikilocytes should be avoided. However, in some situations, there are so many red blood cell shapes or there are some shapes that defy description that the generic term poikilocytosis suffices and provides the same information as identifying each red blood cell shape individually. These situations include physiologic and pathologic poikilocytosis of goats (Vasilitas and Christopher 2023), physiologic leukocytosis of young calves and sheep, chemotherapy-induced poikilocytosis (see image right), and poikilocytosis in cats with liver disease.
The diagnostic relevance of poikilocytes is number-, shape- and context-dependent. Low numbers of misshapen red blood cells can be seen in blood from clinically normal or ill animals and may not be of diagnostic relevance. However, in some situations, low numbers can be helpful. For instance, a few schistocytes in a sick dog with thrombocytopenia should raise suspicion of fragmentation injury, secondary to disseminated intravascular coagulation (DIC).Testing for this hemostastic disorder could be considered, depending on what is wrong with the dog.
The presence of moderate or many misshapen cells is likely to be of more clinical relevance and can actually yield clues as to the cause of anemia in affected animals. For instance, many eccentrocytes and Heinz bodies in an anemic animal indicate oxidant injury and a search for an oxidant (e.g. zinc in dogs, acetaminophen in cats, red maple leaf in horses) should be instituted in such patients. In other instances, many poikilocytes may be an expected or “normal” finding (e.g. certain species of goats, young calves).
In all cases, the fullest interpretation of red blood cell changes can be made only in light of the other parameters of the hemogram, along with pertinent clinical, historical, and laboratory findings. Evaluation for significant shape abnormalities requires a well-made blood film prepared from fresh blood, so that artifactive shape changes are not superimposed upon any of potential pathologic significance.
Acanthocytes
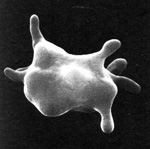
Acanthocytes (or spur cells) are spherical cells with blunt-tipped or club-shaped spicules of different lengths projecting from their surface at irregular intervals. These morphologic changes are most frequently seen in dogs and cats, where they are of diagnostic relevance. They can be seen in other species (cattle, horses) and are of less certain meaning in these species (no specific disease associations have been made with these red blood cell changes). Acanthocytes are less resistant to osmotic lysis and more rigid than normal red blood cells. Due to their rigidity, they probably have a shorter life span than normal, resulting in a mild hemolytic (extravascular) process, which may or may not manifest as an anemia (depending on the number of acanthocytes and the ability of the bone marrow to respond to their premature removal and maintain a normal hematocrit).
Acanthocytes are thought to occur through two main mechanisms:
- Alterations in lipid composition of the red blood cell membrane: This has been mostly documented in liver disease. Studies on human patients and rats with experimentally induced liver disease indicate that the changes in red blood cell shape result from alterations in membrane lipid content secondary to changes in plasma lipids. Increased concentrations of unesterified (free) cholesterol due to production of abnormal lipoproteins (that have a high cholesterol to phospholipid ratio) can occur in various types of liver disease. Unesterified cholesterol in plasma readily exchanges with the cholesterol which is normally found in low concentrations in the red blood cell membranes. In liver disease, the excess plasma cholesterol incorporates preferentially within the outer leaflet of the red blood cell membranes. This makes the red blood cells less deformable and they are remodeled as they passage through the spleen, forming acanthocytes. We speculate that this is the mechanism behind acanthocyte formation in many diseases. For instance, dogs fed a cholesterol-rich diet developed acanthocytes along with an anemia with increased osmotic fragility (Cooper et al 1980).
- Fragmentation injury: Fragmentation can occur because the red blood cells are being damaged as they travel through the vasculature (e.g. being sheared through fibrin clots in disseminated intravascular coagulation; DIC) or they are mechanically fragile (e.g. iron deficiency anemia). In these situations, acanthocytes are frequently accompanied by other red blood cell changes, indicative of fragmentation injury, i.e. schistocytes and keratocytes. However, alterations in cholesterol:phospholipid content in RBC membranes could still be occurring in these diseases.
Acanthocytes are an important finding, because they can provide clues as to the presence of underlying disease in the animal. However, just as for any other shape change, low numbers may not be clinically relevant or diagnostically useful. They can be seen in the following conditions:
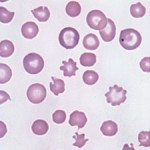
- Normal finding in young ruminants: Calves and kids (generally less than 3 months of age in our experience and documented until 25 days of age in Holstein and Japanese Black Cattle [Sato and Mizuno 1982]) have marked poikilocytes, with many of the red blood cell shapes being compatible with acanthocytes. The mechanism for poikilocytosis in calves is unknown. In goats, it is thought to be due to the presence of an immature hemoglobin, hemoglobin C.
- Disease
- Cancer: In one study, 54% of 123 dogs with acanthocytes had cancer, most commonly hemangiosarcoma, osteosarcoma and lymphoma (usually when the latter tumor involves the liver, in our experience) (Warry et al 2013). The mechanism of acanthocyte formation is unknown. It could be due to alterations in lipid content in the RBC membrane secondary to cancer or cancer infiltrates in the liver causing lipid abnormalities. In dogs with hemangiosarcoma, the acanthocytes are frequently accompanied by schistocytes and thrombocytopenia, suggesting that acanthocytes form secondary to fragmentation injury. Such dogs frequently have laboratory evidence of DIC. Acanthocytes in blood of a dog with hemoabdomen should prompt consideration of underlying hemangiosarcoma (Warry et al 2013). However, acanthocytes in blood do not reliably discriminate between neoplastic and non-neoplastic causes of hemoabdomen in dogs, although platelet counts are lower in dogs with hemangiosarcoma (Wong et al 2015).
- Disseminated intravascular coagulation: Acanthocytes can be seen in low numbers in this disorder, likely as a consequence of fragmentation injury, although schistocytes and keratocytes are more typical. This is most frequently recognized in dogs, but we have seen acanthocytes in cattle with DIC secondary to bacterial sepsis from severe mastitis (along with keratocytes and schistocytes). Animals with DIC are often concurrently anemic and the anemia is usually non-regenerative, due to inflammatory cytokines (anemia of inflammatory disease) associated with the primary disease process that initiates the DIC (e.g. severe inflammation, bacterial sepsis, cancer).
- Iron deficiency anemia: Acanthocytes are commonly observed in the blood of dogs with iron deficiency anemia. Iron-deficient red blood cells are thought to be mechanically fragile, which results in acanthocyte, schistocyte, and keratocyte formation.
- Other disorders:
- Dogs: Acanthocytes can be seen in variable numbers in dogs with different diseases, including cancer as indicated above (osteosarcoma, lymphoma), renal (e.g. acute kidney injury, chronic renal failure), gastrointestinal (gastroenteritis, gastric dilation volvulus), musculoskeletal (e.g. trauma), and cardiac disease (e.g. degenerative valvular disease). Acanthocytes in affected dogs are seen at presentation or develop during hospitalization, supporting changes in plasma lipids as the mechanism for acanthocyte formation (Warry et al., 2013). However, in some dogs, acanthocytes could be due to shearing/fragmentation (e.g. valvular heart disease), concurrent thrombus formation with non-overt DIC (e.g. gastric dilation volvulus), or possibly drug therapy. Acanthocytes are frequently seen in liver disease in cats, e.g. hepatic lipidosis (Christopher and Lee 1994), but are less commonly observed in dogs with liver disease (Warry et al 2013). Affected animals may not be anemic. In some animals, acanthocytes can form due to concurrent DIC, however alterations in the red blood cell membrane may be a contributing mechanism, as described in people. In dogs with liver disease and acanthocytosis, the anemia (if present) is usually mild and reticulocytosis is variable (some have a fairly regenerative anemia, but others are non-regenerative, depending on the cause of the liver disease and any confounding factors which affect the bone marrow response, e.g. inflammation).
- Rabbits: Low numbers of acanthocytes can be seen in rabbits, but rabbits with moderate to many acanthocytes usually have underlying inflammatory or neoplastic disease (Christopher et al 2014).
- Cattle: Acanthocytes and schistocytes have been reported in adult cattle with theileriosis (Stockham et al 2000) (we have also seen these cells in animals infected with different Theileria species, as well as keratocytes)
- Suspect inherited/congenital defect: We and others have seen acanthocytes in individual dogs with no underlying diseases. There is evidence of a compensated hemolytic process in some of these dogs (normal hematocrit, but higher reticulocyte count than normal), indicating the affected cells have decreased red blood cell lifespan. However, such dogs have not developed an overt anemia. A congenital or inherited red blood cell membrane defect is suspected in these dogs. Anecdotally, such “non-pathogenic” acanthocytes have been observed most frequently in Airedale terriers. It has been mentioned that clinically healthy cattle can have acanthocytes (Schalm’s Veterinary Hematology, 4th edition), however it is difficult to accurately distinguish acanthocytes from echinocytes in this species.
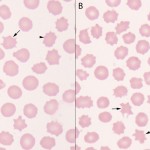
Because they indicate different pathologic processes, acanthocytes must be differentiated from echinocytes, which are also spiculated cells (see images to the right). However the nature of the spiculation, specifically the shape, size and distribution about the membrane differs. The spicules in acanthocytes are more variable in shape (frequently blunt or bulbous ended), vary in size (height above the membrane and width, particularly along the length of the projection) and are unevenly distributed around the membrane. Echinocytes, or crenated red blood cells, in contrast, have shorter, usually sharp blunt spicules of uniform length which are more evenly spaced around their periphery. In some species (those with smaller red blood cells, such as horses and cats), echinocytes may have blunt ends. So in essence, acanthocytes are irregularly spiculated cells and echinocytes are regularly spiculated cells.
Drepanocytes
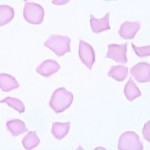
Drepanocytes are sickle-shaped erythrocytes that occurs in certain deer breeds (excluding reindeer and Montjac deer), antelope, some breeds of sheep (e.g. Barbary), goats, antelope, mongoose and genet. The sickling in deer (and probably other species) is an in vitro phenomenon due to variants of hemoglobin (hemoglobin II, alone or in combination with hemoglobin I, III, or IVb) which form insoluble, elongated polymers when in the oxygenated state. The changes are seen in blood exposed for more than a few seconds to atmospheric oxygen, e.g. during mixing of a blood sample or blood smear preparation. Alkalosis can also induce red blood cell sickling, and the in vitro sickling can be prevented by acidification of the blood. Of course, sickling of red blood cells in a human patient is seen in sickle cell anemia, a genetic defect in hemoglobin (a single point mutation in the β-globin gene, in which hydrophilic glutamic acid is substituted for hydrophobic valine, resulting in the formation of a mutant hemoglobin, called hemoglobin “S”). There are mutant mouse models of sickle cell disease, but this hemoglobin defect has not been reported to occur spontaneously in animals.
Eccentrocytes and pyknocytes
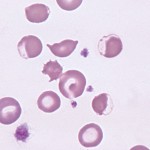
Eccentrocytes form under conditions of excess oxidant stress to the erythrocytes, which induces cross-linking of membrane proteins. Often (but not always), they are seen in association with Heinz bodies, which provide evidence of an oxidant effect on hemoglobin. Eccentrocytes are seen in the following conditions:
- Oxidant-induced hemolytic anemia: Oxidants occur in plants (red maple leaf and Pistacia toxicity in horses [Walter et al 2014], onion poisoning in dogs and cats, Brassica species in ruminants), copper poisoning (sheep), chemicals (zinc pennies in dogs, naphthalene moth balls in dogs, skunk spray in dogs and red pandas [Fierra et al., 2013]) and drugs (phenothiazine drenches in horses, vitamin K1 in dogs). Exposure to these oxidants can result in a hemolytic anemia, which can frequently have an intravascular component.
- Endogenous oxidants: Many sick dogs can have eccentrocytes, indicating oxidant injury to red blood cells (Caldin et al., 2005). This may or may not result in anemia (depending on the extent of oxidant injury).
- Inherited enzyme defects: Inherited defects in enzymes involved in protection against oxidant injury can result in an oxidant-induced hemolytic anemia. Glucose-6-phosphate dehydrogenase deficiency (Stockham et al., 1994) and flavin adenine dinucleotide deficiency (Harvey et al., 2003) have been reported in horses.
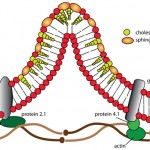
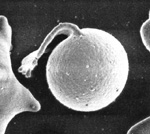
Note that when the thin membrane of the eccentrocyte is removed or ruptures, a small cell which lacks central pallor is formed. These spherocytic cells are termed “pyknocytes” and can be mistaken for spherocytes under light microscopy. Electron microscopy usually reveals the membrane “tags” (whereas spherocytes are uniformly spherical) as shown in the image.
Echinocytes
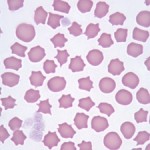
Echinocytes are spiculated RBCs. The term crenation (or “burr” cell) is also used to refer to cells of this type, particularly when the spicules are sharp and the echinocytes are an artifactual change. The projections of the cell membrane may be sharp or blunt, are usually numerous, and tend to be evenly spaced around the circumference. Spicules are usually of uniform size which differentiates echinocytes from acanthocytes (which have irregularly sized spicules, irregularly spaced around the cell membrane). Echinocytes form under the following settings:
- Artifact: Echinocytes usually represent an in vitro artifactive change, resulting from aging of the blood (decreased ATP) and/or exposure to excessive concentrations of EDTA as occurs when the sample tube is significantly underfilled (dehydrates the red blood cells). Echinocyte formation occurs because of red blood cell dehydration, increased pH, and decreased ATP. ATP is required to maintain the shape of the RBC membrane.
- Drugs: Drugs that expand the outer leaflet of the red blood cell membrane produce echinocytosis, e.g. salicylates, phenylbutazone, furosemide, chemotherapeutic agents (e.g. doxorubicin).
- Disorders
- Electrolyte depletion: When seen in fresh, well-handled blood, echinocytes usually indicate whole body electrolyte depletion. Reduction in intracellular erythrocyte potassium (K+) causes red blood cell dehydration and echinocyte formation. This occurs commonly in horses with electrolyte depletion from sweating (endurance horses) or diarrhea (gastrointestinal disturbances such as colitis). Low sodium is the most common electrolyte abnormality seen in blood samples from horses with echinocyte formation, although some horses have no discernible electrolyte changes in blood. (Remember that blood K+ is not a reflection of total body potassium levels.)
- Renal disease: Dogs with renal disease, particularly glomerulonephritis, can have many echinocytes. The mechanism of formation is unknown.
- Inherited red blood cell disorders: Dogs with pyruvate kinase deficiency can have spheroechinocytes. This could be secondary to the decreased ability of affected RBC to produce ATP.
- Snake envenomation: Snake envenomation (rattlesnake, coral snake, vipers) can cause echinocyte formation. Echinocytes can form within 24 hours of the snakebite. This is a useful hematologic marker of this poisoning. Echinocytes are thought to form from phospholipases in the venom, which alter red blood cell membrane phospholipids (Brown et al., 1994, Walton et al., 1997, Hackett et al., 2002).
- Other: Severe burns in horses, Clostridial infections and bee stings have been associated with echinocyte formation.
Unfortunately, because echinocytes are frequently seen as an artifact (usually in stored or “old” blood), they are considered of little pathogenic relevance in most cases. However, they can provide some useful diagnostic information in the appropriate context (those disease conditions listed above) and should not be summarily ignored.
Elliptocytes
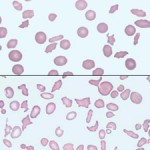
Elliptocytes are elongated red blood cells. There are three types: Type I is a slightly oval-shaped cell (used to be called ovalocyte), type II is a more rounded to oval shaped cell and type III is an elongate elliptical cell. Distinction between these three types is not of clinical relevance, however some forms occur more frequently in some diseases. An occasional elliptical or oval erythrocyte may be seen as a non-specific finding in a variety of settings. In some cases, smear-making technique and/or plasma viscosity may be contributing factors in their in vitro formation. Elliptocytes can indicate underlying diseases, which are listed below.
- Liver disease: One situation in which elliptocytes have some diagnostic specificity is in cats with some forms of liver disease. Many cats with liver disease (particularly lipidosis) have acanthocytes and type III elliptocytes. Some elliptocytes have scalloped or speculated margins and are called oak leaf or holly leaf cells. In some cases, the variation in cell shape is so marked, that specific terms such as acanthocyte and elliptocyte cannot be used (and, in fact, provide no more additional diagnostic information). In these situations, the generic term poikilocytosis is used and confers the same meaning as identifying each shape change individually (Christopher and Lee 1994).
- Myelofibrosis: Elliptocytes (type 1 or ovalocytes) in dogs should raise suspicion of underlying myelofibrosis. Such elliptocytes are usually seen with non-regenerative forms of immune-mediated anemia (NRIMA) or (presumptive) precursor-directed immune-mediated anemia (PIMA), as well as dogs with pure red cell aplasia (the most severe form of NRIMA). Myelofibrosis can also occur with primary hematopoietic neoplasia and cancers that metastasize to the bone marrow (lymphoma, histolytic sarcoma).
- Inherited/congenital red blood cell abnormality: A defect in the red blood cell membrane 4.1 (Smith et al., 1983) or the cytoskeletal protein, spectrin (Terlizzi et al., 2009), can result in elliptocytosis.
- Chemotherapy: Elliptocytes have been observed in cats given doxorubicin (O’Keefe and Schaeffer 1992). Keratocytes were also identified in cats in the latter study, however keratocytes can be seen in blood films from cats that were not given doxorubicin and are clinically healthy (Mike Scott, personal communication).
Keratocytes
Keratocytes are erythrocytes with a blister-like vesicle, which may rupture, leaving a “bite-shaped” defect in the cell outline or one or two horn-like projections on the same side of the cell. This process may occur more than once for a given cell, resulting in very irregular shapes.
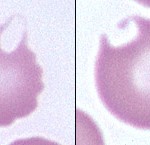
Low numbers of keratocytes may be seen in various situations and may not have any clear clinical significance (e.g. healthy cats may have a few keratocytes in blood). When present in larger numbers or with other poikilocytes, keratocytes can indicate the following:
- Fragmentation injury: Keratocytes will usually accompany schistocytes and acanthocytes in this setting, Associated conditions include causes of microangiopathic hemolysis (disseminated intravascular coagulation, vasculitis, hemangiosarcoma) and mechanical fragility, e.g. iron deficiency anemia.
- Oxidant injury: Here keratocytes may accompany eccentrocytes, pyknocytes, and possible Heinz bodies, depending on the oxidant.
- Liver disease: In cats, keratocytes can be seen in increased numbers in liver disease, e.g. hepatic lipidosis. The mechanism is unclear and could be related to mechanical fragility from alterations in phospholipid or cholesterol composition of the red blood cell membrane (membrane rigidity) or disseminated intravascular coagulation.
Poikilocytes
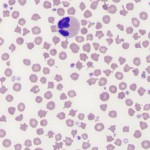
This generic term is used when the red blood cell shape changes are so varied, that using specific terms does not provide additional diagnostic meaning. The term is rarely used in dogs (because we can usually identify the specific shape change, such as acanthocyte, which has more diagnostic meaning than the generic term of poikilocyte) and is usually only applied to the following species (and situations):
- Physiologic poikilocytosis: Marked poikilocytosis can be a normal feature in the blood of some goats (especially Angora and Boer) and is especially prominent in kids (< 3 months of age), although poikilocytosis can be seen in older goats, up to 1 year of age. The prevalence of subjectively graded moderate to marked poikilocytosis in one study of 1254 goats was 46% in animals under a year of age (Vasilatis and Christopher 2023). In the same study, poikilocytosis is also present in adult goats with a prevalence of 19% and was identified in non-anemic and anemic goats. Described poikilocytes were dacryocytes, elliptocytes, schistocytes, “matchstick”, acuminocytes (fusiform), acanthocytes (spiculated) and polygonal (e.g. triangular) forms. The hematocrit was slightly but significantly lower in juvenile (6-12 months of age) and adult (≥1 year of age) goats with moderate to marked versus none to slight poikilocytosis, suggesting that poikilocytes do have reduced life span. Adult goats with moderate to marked poikilocytosis had significantly but marginally lower MCV (18.6 fL) versus those with none to slight poikilocytosis (19.4 fL) and a marginally increased MCHC (34.4 vs 34.8 g/dL, respectively). Goats with Johnes disease, gastrointestinal (GI) parasitism, and lice infestation had lower hematocrits (20-25% versus 29%), MCV (17-18 fL versus 20 fL), and similar or higher MCHC (34-35 g/dL versus 34 g/dL) than healthy goats and also had a higher proportion of individuals with moderate to marked poikilocytosis. The lowest hematocrit and MCV was seen in goats with Johne’s disease (Vasilatis and Christopher 2023). The authors attributed the poikilocytosis and low MCV to iron deficiency, however the association between poikilocytosis and MCV was poor (Spearman rank correlation of <0.3), iron concentrations were not measured, hypochromic red blood cells were not described as a blood smear change, and the increased MCHC is not expected in iron deficiency. Poikilocytes may have reduced volume, which might partly explain the lower MCV. It is possible that the poikilocytosis reflects different variants of hemoglobin production versus iron deficiency, which may better explain the MCHC changes. A few goats in the study had Haemonchus contortus infections and likely had iron deficiency anemia, however these were not separated out from the other goats with GI parasitism, where iron deficiency from blood loss is not expected. The anemia in goats with lice infestation and GI parasitism likely reflects anemia of inflammatory disease, which is associated with iron sequestration (and has been associated with a functional iron deficiency in people, which may result in microcytic red blood cells over time). Additional information on goats: Their red blood cells are among the smallest of the domestic animal species, with MCVs ranging from 16-25 fL (Barbary sheep have lower MCVs). The caprine erythrocyte lifespan is approximately 125 days. Goats lack polychromasia in peripheral blood in health but do release polychromatophils into circulation in response to an anemia (macrocytes can also be seen).
Marked poikilocytosis, thrombocytosis (often > 1 million cells/uL) and microcytosis are features of healthy calf blood (usually under 3 months of age), with the latter finding being attributed to a “physiologic” iron deficiency (some calves may also have mildly hypochromic red blood cells in a smear; see above image). Calves can remain microcytic for up to 1 year of age. - Liver disease in cats: Moderate to marked poikilocytosis in a non-anemic or anemic cat is a good non-invasive marker for underlying liver disease (most frequently, but not limited to, lipidosis).
- Drugs: Certain drugs, e.g. doxorubicin, can induce poikilocytosis.
Schistocytes
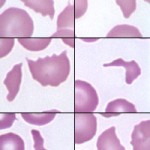
Schistocytes, or red blood cell fragments, are generally taken to reflect mechanical injury to erythrocytes. A wide variety of forms may be observed.
Usually seen in relatively low numbers, they are associated with conditions in which the normally smooth endothelial lining is roughened or irregular, the vascular lumen is crossed by strands of fibrin, or blood flow is turbulent. Disseminated intravascular coagulation, glomerular disease, vasculitis, portosystemic shunts and vascular neoplasms (e.g. hemangiosarcoma) are among the many conditions in which red blood cell fragmentation may be seen in varying degrees. Schistocytes are seen in up to 60-75% of dogs, but are rarely seen in cats and other species, with disseminated intravascular coagulation (in cats, schistocytes are most commonly observed as part of the poikilocytosis in liver disease).
Schistocytes also are commonly seen as a feature of iron deficiency anemia, but in this instance the fragmentation likely results from inherent mechanical fragility of the cells produced under these conditions than from changes in the vasculature.
Spherocytes
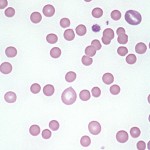
Spherocytes are erythrocytes which have assumed the form of a sphere rather than the normal discoid shape. As a result, they appear on routine blood films as cells that are smaller and more dense than normal red blood cells of the species, and have a reduced area of central pallor. Because cats, horses and cattle normally have red cells with little central pallor, recognition of spherocytes is more difficult in these species than in dogs, whose normal red blood cells have distinct pale centers. Note that even though spherocytes appear smaller than normal red blood cells on a blood smear (because you are assessing diameter of cells on a smear, not volume), in most cases, they have normal volume and do not change the mean cell volume (MCV).
There are several causes of spherocyte formation and numbers do matter to some extent. Moderate to marked spherocytosis is diagnostic of immune-mediated hemolytic anemia (IMHA). Low numbers of spherocytes can be seen in conditions other than IMHA, therefore the presence of spherocytes (especially if in low numbers) is not always indicative of IMHA.
- Immune-mediated hemolytic anemia: Moderate to marked spherocytosis is characteristic of this disorder, although low numbers can be seen in some dogs and others may have no spherocytes at all. Some studies show that up to 66% of dogs with IMHA have spherocytosis, however in our experience, this number is higher (approaching 90% or more) in dogs with classic regenerative IMHA. Spherocytes are not as frequently seen in non-regenerative forms of immune-mediated anemia or the precursor-directed immune-mediated anemias. In classic regenerative IMHA, spherocytes are thought to be produced when antibody coating red blood cells bind to the Fc portion of macrophages, resulting in partial phagocytosis of the red blood cell. The remnant has a reduced surface area to volume ratio and assumes a sphere shape rather than a disc. A positive direct Coombs’ test (also called direct antiglobulin test or DAT) or detection of spontaneous three-dimensional clumping of red blood cells (autoagglutination) confirms the presence of antibody on red blood cells.
- Other acquired diseases: These usually have low numbers of spherocytes and include fragmentation anemias, oxidative injury to red blood cells (although the cells are actually pyknocytes), coral snake envenomation, pyruvate kinase deficiency in Basenjis (spheroechinocytes) and disorders associated with abnormal macrophage function (hemophagocytic syndrome, histolytic sarcoma), and bee stings.
- Inherited red blood cell abnormality: Japanese black cattle can have a defect in erythrocyte membrane protein band 3, which results in hereditary spherocytosis (Inaba et al., 1996).
- Transfused or stored red blood cells: Stored red blood cells in blood bags will lose surface area with storage and, when transfused, will appear as spherocytes in blood smears from the recipient. In this situation, moderate to many spherocytes may be seen, depending on the ratio of transfused donor cells to the patient’s own cells.
- “Normal” finding: A few spherocytes are frequently seen at or near the feathered edge of blood smears in many dogs (normal or ill) and should not be over-interpreted. Their presence should be verified by inspection of red blood cell morphology in the monolayer and deeper areas of the smear.
Stomatocytes
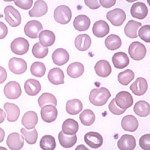
Stomatocytes are erythrocytes with an elongated (mouth-like) area of central pallor. An occasional cell of this type might be seen as a non-specific finding in a variety of situations, such as regenerative anemias, liver disease, and lead poisoning. Stomatocytes can also be an artifact in a blood smear that is too thick. They can also be a marker of inherited red blood cell defects in dogs.
- “Normal” finding: Woodchucks and some aquatic animals (manatees, dolphins) can normally have stomatocytes.
- Hereditary stomatocytosis: This occurs in some Alaskan Malamute dogs in association with chondrodysplasia, manifesting as dwarfism. It is inherited as an autosomal recessive trait. In addition to the morphologic abnormality, the red cell population in homozygous affected dogs is macrocytic (up to 98 FL), and hypochromic (26-28%). Erythrocyte numbers are lower than normal, but, due to the larger cell size, hematocrits are within reference intervals. Red blood cell lifespan is reduced, with extravascular hemolysis and a mild reticulocytosis, and the cells are osmotically and mechanically fragile. The precise defect is unknown, but it is a membrane defect involving increased sodium and water influx.
Hereditary stomatocytosis has also been reported in Drentsche Patrijshond (in conjunction with hypertrophic gastritis) and Standard (Bonfanti et al., 2005) and Miniature Schnauzers (it appears autosomal recessive in the latter). We have seen one case each of stomatocytosis in a Peek-a-poo and Pomeranian at Cornell University. In the Drentsche Patrijshond, dogs are anemic (Hct, 18-38%) with reticulocytosis (2.4-12%), macrocytic (72-81 fL) and hypochromic. Stomatocytes are seen in 14-38% of red blood cells by light microscopy. The defect is due to abnormal phospholipid composition in the red blood cell membrane (increased sphingomyelin and decreased cholesterol and phosphatidylcholine, with the latter being the most consistent change). The dogs usually die of hemolytic anemia.
Target cells
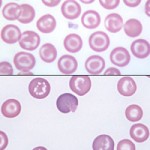
Target cells (codocytes or leptocytes) have a “lump” of hemoglobinized cytoplasm within the area of normal central pallor, causing them to resemble a “bullseye” target. The ability to assume this form is said to reflect an increased surface to volume ratio. Their deformability is similar to that of normal cells and they are more resistant to osmotic lysis. These cells are only really observed in dogs.
Young erythrocytes, as a normal feature, have excess membrane relative to mature cells; therefore, polychromatophilic target cells are often seen in the context of a regenerative anemia. Hypochromic cells, in iron deficiency anemias, also can assume a target appearance because the cells have larger diameters with decreased cell thickness. In these situations, the presence of target cells in a blood smear does not provide any additional diagnostic information.
Increased numbers of normochromic target cells can be a useful diagnostic indicator of pathologic conditions resulting in a balanced increase in cholesterol and phospholipid in the redblood cell membrane. The most common disorder associated with normocytic normochromic target cells is liver disease. Hypothyroidism is another condition where this can occur and could be related to high systemic cholesterol concentrations that are seen in this disorder in dogs.
