Interpretation
Parasitic (trematode) infection
Explanation
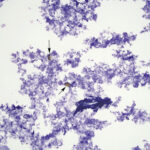
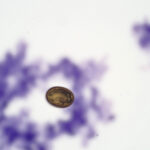
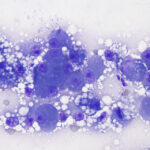
The direct smears of bile consisted of variably sized small granules of blue-gray bile, with no inflammatory cells. There were also low numbers of smooth yellow-brown cup-shaped parasitic ova, which were 40-50 μm long and 20-30 μm wide (Figures 1a, 2, and 3). The ova had a single operculum (difficult to see in the images) and internal complexity (Figure 3). The ova had features consistent with the trematode, Eurytrema procyonis1 (Question 4). Liver aspirates revealed numerous small vacuoles within hepatocytes, consistent with hepatic lipidosis (Figure 4).
 |
 |
 |
The increased ALT activity indicated hepatocellular injury, likely secondary to the lipidosis, whereas the hyperbilirubinemia with bilirubinuria (always abnormal in cats) and increased ALP activity indicated cholestasis, with increases in direct bilirubin (a bilirubin split was not done, but the hyperbilirubinemia was likely due to a combination of both direct and indirect bilirubin). The absence of bile casts in the liver aspirate can be explained by sampling of areas without cholestasis. Lipidosis will result in enlarged hyperechoic livers2 and typically causes increased activities of ALT, AST, and ALP, with hyperbilirubinemia, mostly due to increases in direct bilirubin in cats3 (Question 1). The hyperglycemia and glucosuria supported concurrent diabetes mellitus with ketosis (based on the ketonuria). Diabetes mellitus can cause hepatic lipidosis due to alterations in lipid metabolism (Question 2). Insulin stimulates the activity of endothelial lipoprotein lipase, which breaks down chylomicrons and very high density lipoproteins (VLDL) to free nonesterified fatty acids and glycerol for energy use by tissues. Insulin also opposes the activity of hormone-sensitive lipase, which causes lipolysis of fat stores, liberating free fatty acids, which are transported to the liver for energy use and packaging into VLDL. Lack of insulin would result in increased lipolysis (due to unopposed hormone-sensitive lipase activity, with the latter being stimulated by “stress” hormones, such as glucocorticoids) and more delivery of free fatty acids to the liver. The fatty acids that cannot be used for energy production or that are not exported as VLDL will be shunted into ketone production (explaining the ketosis in this cat) and stored in the liver, resulting in lipidosis. Cats with hepatic lipidosis are frequently inappetent or anorexic; the resulting negative energy balance also liberates fat stores and is a predisposing factor for hepatic lipidosis, even in the absence of diabetes mellitus. The unexpected biochemical result in this cat is the high GGT activity, because GGT activity is usually normal in cats with hepatic lipidosis.3 However, animals with longer-standing lipidosis or other processes involving the liver, potentially increased biliary pressure from a fluke in the bile duct, could be responsible for the high GGT activity in this cat (Question 3).
Follow up
Endoscopic assessment of the gastrointestinal tract revealed mild gastric thickening with bleeding ulcers and a mildly thickened duodenum with raised white to yellow plaques. Histologic evaluation of endoscopic biopsies revealed moderate neutrophilic and ulcerative gastritis and enteritis with underlying lymphoplasmacytic enteritis. A zinc sulfate and sugar flotation test on feces yielded eggs, consistent with Eurytrema procyonis. The cat was treated with insulin for the diabetes mellitus and praziquental for the fluke infection. A repeat fecal flotation done 4 years later was negative for trematode eggs and the cat’s diabetes mellitus was successfully controlled with insulin (last recheck 5 years after the diagnosis of the trematode infection).
Discussion
Eurytrema procyonis is a trematode parasite that resides in the pancreatic ducts of raccoons, foxes and cats.1,4-12 It can, however, be found within the common bile duct,3 which was considered likely in this cat, based on the finding of trematode eggs in an aspirate of bile. In one study of 154 domestic cats, trematode infections were uncovered by fecal testing in 6 cats (14%) and infected cats had up to 1800 flukes.5 However, the true prevalence of infection and lifecycle of the organism remains unknown. Trematode eggs harvested from a gravid worm can establish a productive infection in the common garden snail (Mesodon thyroidus), which may serve as the intermediate host.1 After ingestion of the intermediate host, the flukes penetrate the duodenum or may migrate up the pancreatic duct. Once within the ducts, the flukes attach to the walls, causing ductular dilation and thickening, with periductular fibrosis and monononuclear and eosinophilic inflammation.5,7,10,11 Sloughing of ductular epithelial cells and epithelial hyperplasia can also be seen and in some animals, the fibrosis and inflammation can extend into adjacent pancreatic tissue,5,9 with resulting pancreatic atrophy.5,9-11 Duct obstruction with decreased bicarbonate excretion can be seen with heavy worm burdens.9 Hemograms and biochemical profiles are often normal in affected cats,8 with no changes in amylase or lipase activity,5,8 despite the chronic fibrosing pancreatitis. Serum pancreatic lipase immunoreactivity may be increased in some cats.8 Ultrasonographic examination of affected cats may reveal dilated thickened pancreatic ducts and features of pancreatitis,8 such as an enlarged hypoechoic pancreas with hyperechoic peripancreatic regions.8,13 In this case, the changes in the biochemical profile were attributed to lipidosis versus the trematode infection and there was no ultrasonographic evidence of pancreatic duct dilation or pancreatitis (Question 5). The infection is typically clinically asymptomatic; non-specific signs of weight loss and vomiting have been reported in one cat, potentially secondary to the pancreatitis, although co-morbidities were not ruled out.11 In this case, the weight loss was likely secondary to the gastroenteritis and diabetes mellitus than the fluke infection. Similarly, the fever could be attributed to the ulcerative and neutrophilic gastroenteritis. Since the cat had been housed indoors for 3 years, it had likely acquired the trematode infection from ingestion of a snail, while living on the farm.
The diagnosis of trematode infection in this case was made from an aspirate of bile and confirmed by fecal flotation. Typically, the infection is identified in living animals via the detection of the characteristic eggs on fecal flotation.5,10 There has been one previous report of a cytologic diagnosis of Eurytrema infection being made from an aspirate of pancreatic nodules discovered on exploratory laparotomy of a cat that was presented with a short history of anorexia, gagging, and painful abdomen. The affected region of pancreas was the tail and it was described as being “thickened, nodular, and dark brown to black”. Trematode eggs and mixed eosinophilic and neutrophilic inflammation was diagnosed on cytologic assessment, but histologic evaluation of the pancreas was not done.12 The main differential diagnosis for the parasitic ova is the liver fluke, Platynosomum fastosum, which has similar eggs, but causes liver disease and is found in tropical or sub-tropical regions, such as Florida in the US. In a previous report of liver fluke infection, ova and suppurative inflammation was found on cytologic evaluation and changes in liver enzyme activities and bilirubin concentrations were more pronounced.14 Considering the cat originated from the tropical island of St. John, one could speculate that it was chronically infected with the liver fluke, Platynosomum, and did not have Eurytrema infection. Cats harboring a liver fluke infection can be asymptomatic or suffer from clinical liver disease and even failure.15,16 Ultimately, discrimination between these two parasites would require examination of the adult worms and histologic examination of the liver, gall bladder and pancreas at the time of infection, which was not done in this case.

Aspirates of bile usually consist of granular blue-gray pigment, as seen in this case, although bilirubin crystals (variably sized slender needles or granules) or chunks of yellow-brown bile may be observed. Columnar epithelial cells, likely of biliary ductal origin, are seen in a few cases. Abnormal findings, other than trematode eggs, include bacteria (representing an ascending infection or cholecystitis) with or without inflammatory cells, inflammatory cells, and hemorrhage.17,18 Tachyzoites of Isospora coccidia and the intestinal fungus, Cyniclomyces guttulatus, are considered incidental findings.17,19 We have also seen cases of “white bile”, where the pigment granules are absent and only mucus is present (Figure 5). In a few cases, the bile pigment is found in thick concretions, suggesting dehydration or inspissation of bile. Bacteria isolated from bile are typically enteric organisms, with Escherichia coli and Enterococcus being the most common isolates in both dogs and cats.17 Subclinical bactibilia can occur in around 10% of clinically healthy dogs (diagnosed by culture or cytologic assessment of bile) and affected dogs do not have more histopathologic abnormalities than dogs without bactibilia (although intermittent bactibilia may have been missed in the latter dogs).20
References
- Denton JF. Studies on the Life History of Eurytrema procyonis Denton, 1942. J Parasitol. 1944;30(5):277-286. doi:10.2307/3272577
- Griffin S. Feline abdominal ultrasonography: what’s normal? what’s abnormal? The liver. J Feline Med Surg. 2019;21(1):12-24. doi:10.1177/1098612X18818666
- Center SA, Crawford MA, Guida L, Erb HN, King J. A retrospective study of 77 cats with severe hepatic lipidosis: 1975-1990. J Vet Intern Med. 1993;7(6):349-359. doi:10.1111/j.1939-1676.1993.tb01030.x
- Herman CM, Bauman PM, Habermann RT. The prevalence of Eurytrema procyonis Denton (Trematoda: Dicrocoeliidae) in some mammals from Maryland. J Parasitol. 1957;43(1):113-114.
- Carney PC, Ruaux CG, Suchodolski JS, Steiner JM. Biological variability of C-reactive protein and specific canine pancreatic lipase immunoreactivity in apparently healthy dogs. J Vet Intern Med. 2011;25:825-830. doi:10.1111/j.1939-1676.2011.0729.x
- Richardson DJ, Owen WB, Snyder DE. Helminth parasites of the raccoon (Procyon lotor) from north-central Arkansas. J Parasitol. 1992;78(1):163-166.
- Foley GL, Anderson WI, Georgi ME. Eurytrema procyonis in a New York fox. Cornell Vet. 1987;77(2):168-171.
- Vyhnal KK, Barr SC, Hornbuckle WE, et al. Eurytrema procyonis and pancreatitis in a cat. J Feline Med Surg. 2008;10(4):384-387. doi:10.1016/j.jfms.2008.01.001
- Fox JN, Mosley JG, Vogler GA, Austin JL, Reber HA. Pancreatic function in domestic cats with pancreatic fluke infection. J Am Vet Med Assoc. 1981;178(1):58-60.
- Sheldon WG. Pancreatic flukes (Eurytrema procyonis) in domestic cats. J Am Vet Med Assoc. 1966;148(3):251-253.
- Anderson WI, Georgi ME, Car BD. Pancreatic atrophy and fibrosis associated with Eurytrema procyonis in a domestic cat. Vet Rec. 1987;120(10):235-236. doi:10.1136/vr.120.10.235
- Hoffman DA, Piech TL, Taylor HL, Royal AB. What is your diagnosis? Pancreatic aspirate from a cat. Vet Clin Pathol. 2017;46(3):540-541. doi:10.1111/vcp.12501
- Nivy R, Kaplanov A, Kuzi S, et al. A retrospective study of 157 hospitalized cats with pancreatitis in a tertiary care center: Clinical, imaging and laboratory findings, potential prognostic markers and outcome. J Vet Intern Med. 2018;32(6):1874-1885. doi:10.1111/jvim.15317
- Stern JK, Walden HDS, Marshall K, Sharkey L. What is your diagnosis? Bile from a cat. Vet Clin Pathol. 2020;49(2):354-355. doi:10.1111/vcp.12840
- Basu AK, Charles RA. A review of the cat liver fluke Platynosomum fastosum Kossack, 1910 (Trematoda: Dicrocoeliidae). Vet Parasitol. 2014;200:1-7.doi: 10.1016/j.vetpar.2013.12.016
- Taylor D, Perri SF. Experimental infection of cats with the liver fluke, Platynosomum concinnum. Am J Vet Res. 1977;38:51-54.
- Peters LM, Glanemann B, Garden OA, Szladovits B. Cytological Findings of 140 Bile Samples from Dogs and Cats and Associated Clinical Pathological Data. J Vet Intern Med. 2016;30(1):123-131. doi:10.1111/jvim.13645
- Palić J, Geisen V, Unterer S, Dorsch R. The beauty of bile. Veterinary Clinical Pathology. 2017;46(4):549-550. doi:10.1111/vcp.12525
- Neel JA, Tarigo J, Grindem CB. Gallbladder aspirate from a dog. Vet Clin Pathol. 2006;35:467-470.
- Verwey E, Gal A, Kettner F, Botha WJ, Pazzi P. Prevalence of subclinical bactibilia in apparently healthy shelter dogs. J Small Anim Pract. Published online July 15, 2021. doi:10.1111/jsap.13398
Authored by: T Stokol and M Forman.
