Interpretation
Copper accumulation
Explanation
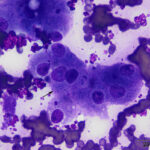
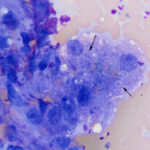
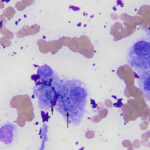
The aspirate contained moderate to many hepatocytes with overlying ultrasound gel (Figures 1-3). Hepatocytes were uniform in appearance, with low numbers containing a few discrete margined vacuoles, compatible with lipid (Figure 2). A few cells were also binucleated (Figure 1). Small to moderate amounts of blue-green pigment, which was slightly refractile (crystalline) when focusing up and down, was noted in several hepatocytes (Figures 1-3, Question 1). The pigment could be lipofuscin, however the slightly refractile nature was more compatible with copper. Neither a discernible inflammatory infiltrate nor bile casts were seen on scanning the slides.
 |
 |
 |
Additional tests
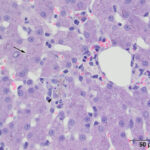
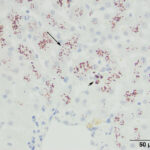
A wedge biopsy of the liver was taken at the same time as the liver aspirate and submitted to the Anatomic Pathology service for histopathologic examination. A moderate amount of red-brown pigment was seen within hepatocytes in all zones in hematoxylin & eosin-stained sections of the liver (Figure 4). The same pigment was also present in macrophages, which formed focal aggregates. Low numbers of other inflammatory cells (lymphocytes, plasma cells) were seen with the macrophages and in the portal and central vein regions. The histologic diagnosis was moderate multifocal granulomatous hepatitis with copper accumulation and mild central venous and portal lymphoplasmacytic inflammation. Based on the young age of the cat and the abundant copper, a primary copper hepatopathy was suspected. A rhodanine stain was performed on a section of the liver to highlight the copper. This stain showed abundant intracytoplasmic copper, mostly within zone 3 hepatocytes (centrilobular area) with smaller amounts in hepatocytes in zone 1 (portal region), with multifocal copper granulomas. The copper was quantified digitally at 1,779 μg/g dry weight (normal < 400 μg/g).
 |
 |
Discussion
Copper toxicity is a well-recognized cause of liver disease in the dog, with several breeds, such as Bedlington Terriers, West Highland White Terriers, Labrador Retrievers and Dobermans, having a genetic predisposition to develop a primary copper hepatopathy (see June 2017 case of the month). However, two retrospective studies demonstrate that copper does accumulate in the liver of cats, including those with presumed primary copper hepatopathy and those with other liver diseases, such as cholangitis-cholangiohepatitis and extrahepatic bile duct obstruction.1,2 In one of these retrospective studies, involving hepatic biopsies from 104 cats, 11% (12 cats) had evidence of copper accumulation (confirmed with rubeanic acid staining) and all had additional hepatic histologic abnormalities, including glycogen accumulation, cholangitis-cholangiohepatitis, portal hepatitis, lipidosis, neoplasia (lymphoma, cholangiocarcinoma), pyogranulomatous hepatitis due to feline infectious peritonitis, and a portosystemic shunt. In the second retrospective study of 100 cats, cats with presumptive primary copper hepatopathy (n=11) were distinguished from those with secondary copper accumulation, on the basis of high concentrations of hepatic copper (>700 μg/g dry weight as determined by atomic mass spectrophotometry or digital quantification from rhodanine-stained slides), localized to the centrilobular (zone 3) or midzonal and centrilobular regions (zones 2 and 3), with no evidence of another disease causing cholestasis. There also have been a few case reports of cats with primary copper hepatopathy.3–5 In one of these reports, two single nucleotide polymorphisms were identified in the ATP7B gene, which is a membrane-spanning ATP-dependent copper shuttle within cells. One of the polymorphisms was highly conserved and predicted to result in a dysfunctional protein based on its location within the ATP binding domain. Since the ATP7B gene product is involved in biliary copper excretion, its dysfunction would result in copper accumulation. Genetic defects in this protein are associated with Wilson’s disease, a primary copper storage disease in humans.6 Based on the young age of the cat in this report and the marked copper accumulation in zones 2 and 3 of the liver on histologic evaluation, it is likely that the cat had a primary copper hepatopathy.
Cats with presumptive primary copper hepatopathy are typically young (2 years of age or younger) and present with non-specific clinical signs, including decreased appetite, anorexia, weight loss, and vomiting .1,3–5 Some cats are icteric, providing clinical evidence of liver disease.1,3,5 Several cats had liver biopsies performed due to persistently increased liver enzymes.1 Signs can be present for days to years.1,3,5 Biochemical testing yields evidence of liver injury, with high liver leakage enzyme activities (ALT, aspartate aminotransferase [AST] and lactate dehydrogenase). Activities of ALT tend to be higher than AST.1,3 Some cats have hyperbilirubinemia and increased cholestatic enzyme activity1,3,5 (alkaline phosphatase to a greater extent than γ-glutamyl transferase1). Although these biochemical changes do not distinguish copper hepatopathy from other liver disorders, increased liver enzymes in a young cat should raise suspicion for a primary copper hepatopathy, prompting considerations for a liver biopsy. Most cats lack biochemical of hepatic failure (normal urea nitrogen, cholesterol and albumin concentrations; concentrated urine),1,3 although 2 cats were stated to be in liver failure in one retrospective study1 and one other cat had high fasting ammonia concentrations.5 One cat developed a Heinz body hemolytic anemia, which was attributed to hepatic necrosis with release of copper causing oxidant injury. A hemolytic anemia is also seen in Bedlington Terriers with copper hepatopathy.7
Ultrasonographic examination of the liver of cats with presumptive primary copper hepatopathy did not yield specific findings, although 2 cats had coarse hepatic echogenicity,1 which was also seen in the cat in this report. However, coarse echogenicity is not unique to this disorder, being identified in cats with cholangitis-cholangiohepatitis and miscellaneous hepatic disorders.1
There have been no prior cytologic descriptions of copper in aspirates of the liver of cats, although assessment for copper has become a standard part of the diagnostic evaluation of liver aspirates from dogs. Copper is typically seen as turquoise or sea-green refractile inclusions within the cytoplasm of hepatocytes on modified Wright’s-stained smears and is most evident when there is moderate to marked accumulation. It can be readily missed if accumulation is mild or the copper is present in low numbers of hepatocytes. In addition, when there is non-uniform zonal accumulation, involved hepatocytes may be missed on aspiration or cytologic examination of the smear. In this case, there were only low numbers of hepatocytes which contained a small number of typical copper granules in the cytoplasm, with many hepatocytes lacking pigment or much of the pigment being darker and less refractile. Classic features of copper granules were also more difficult to see in the Diff-quik-stained smear. The darker pigment was interpreted as lipofuscin on initial examination of the liver aspirate (and the more classic copper granules were missed), however, lipofuscin would be unusual in the liver of such a young cat. Lipofuscin is considered a “wear-and-tear” pigment (presumably from oxidative or other forms of injury) and is more common in the livers of older cats.8 Another pigment that can be seen within hepatocytes is iron, however this is usually present in small amounts2 and is not generally discernible in Romanowsky-stained cytologic smears.
Due to the difficulties in recognizing small amounts of copper in some cases and potential for misidentification of other pigments as copper, a copper-specific stain, such as rhodanine, can be performed on previously stained cytologic smears to confirm copper accumulation. In our experience, such stains have highlighted small amounts of copper that were not detected in some hepatocytes in routinely stained smears of liver aspirates in dogs. Rhodanine staining was not done on the cytologic smears in this particular case, because the staining was performed on the hepatic biopsy and was conclusive for copper. Even if copper is observed in cytologic smears, hepatic biopsy is always recommended to assess for degree and zonal location of the copper. The latter architectural features can help distinguish between presumptive primary and secondary copper accumulation. In cats with presumptive primary copper hepatopathy, copper was concentrated in zone 3 and extended into zone 2,1,3,4 similar to that seen in dogs with primary copper hepatopathy. In contrast, copper was primarily found around portal tracts in cats with extrahepatic bile duct obstruction and, when evident, was located in small amounts in random locations in cats with low concentrations of copper (<180 μg/g dry weight).1
Aside from copper accumulation, cats with presumptive primary copper hepatopathy can have mixed inflammatory infiltrates (often around the central vein) and copper within macrophages, which infrequently form aggregates.1,3–5 Copper also seems to be associated with hepatic fibrosis.1,2,4,5 Most of the reported cases have also shown hepatocellular features consistent with glycogen accumulation,1,3,4 which is an unusual form of vacuolar change in feline livers, with lipid being more common. Indeed, in the retrospective study of 104 cats in which hepatic iron and copper was assessed,2 it is likely that the one cat with large amounts of copper in the centrilobular zone and glycogen accumulation had a primary copper hepatopathy, even though it was not categorized as such. Cytoplasmic rarefaction compatible with glycogen accumulation (indistinct wispy vacuolation versus the discrete margined vacuoles of lipid) was not evident in the hepatocytes in this case on cytologic or histologic examination, suggesting that this is not an invariable finding in cats with primary copper hepatopathy. However, glycogen accumulation could have been present in non-sampled regions of the liver. Thus, in cytologic smears, evidence of fibrosis (e.g. excess matrix, spindle cells) or glycogen accumulation should prompt a search or special stain for copper. Other histopathologic findings in some cases include individual hepatocellular necrosis and nodular regeneration3,5 and copper accumulation in the renal proximal convoluted tubules and collecting ducts and alveolar macrophages epithelium.3
High concentrations of copper (defined as >700 μg/g dry weight1) were found in the liver of cats with presumptive primary copper hepatopathy (median 1,830 μg/g dry weight, range 704-7,041 μg/g dry weight)1, with many having concentrations >4,000 μg/g dry weight.1,3,4 However, several cats with secondary hepatic copper accumulation due to extrahepatic bile duct obstruction, cholangitis-cholangiohepatitis and various hepatocellular diseases (e.g. hepatic lipidosis, portosystemic shunts, lymphoma, metastatic carcinoma) had copper concentrations above the defined cut-off concentration.1 Cats without hepatobiliary disease had far lower hepatic copper concentrations (<210 μg/g dry weight).1,3,4 Considering that cats with extrahepatic bile duct obstruction had high copper concentrations (median 238 μg/g dry weight, range 63-1,004 μg/g dry weight), it does appear as if cholestasis will result in secondary copper accumulation in cats. The copper is found in the portal region (zone 1) and may extend into the intermediate zone in cats with cholestasis. In contrast, copper does not appear to reliably increase in the liver of dogs with bile duct obstruction,9,10 unless they are concurrently given copper parenterally.9 Aside from likely genetic defects in proteins involved in copper metabolism or excretion, diet appears to be the main influence of liver copper concentrations in both dogs and cats.11,12
Cats with presumptive primary copper hepatopathy have been treated with supportive therapy, including hepatic-based diets, choloretics and antioxidant drugs. Some cats have been given corticosteroids to combat concurrent inflammation. Several cats have been treated with chelating agents, such as D-penicillamine, and have shown decreases in liver enzyme activities after treatment, supporting a response to therapy.1,5 However, D-penicillamine appeared to cause a hemolytic crisis in 1 cat, necessitating withdrawal of therapy.1
In conclusion, this case indicates that, although uncommon, copper does accumulate in the liver of cats with various diseases and that cases of primary copper hepatopathy do occur. Copper can be readily overlooked on cytologic evaluation. The index of suspicion for a primary copper hepatopathy should be increased in a younger animal with persistently increased liver enzyme activities, with or without hepatic ultrsonographic abnormalities or if glycogen-type vacuolar change or fibrosis are evident or suspected in cytologic smears. If a pigment within hepatocytes is concerning for copper, staining of prestained or unstained smears for copper (rhodanine, rubeanic acid) would be worthwhile.
References
- Hurwitz BM, Center SA, Randolph JF, et al. Presumed primary and secondary hepatic copper accumulation in cats. Journal of the American Veterinary Medical Association 2014;244:68–77.
- Whittemore JC, Newkirk KM, Reel DM, et al. Hepatic copper and iron accumulation and histologic findings in 104 feline liver biopsies. J Vet Diagn Invest 2012;24:656–661.
- Haynes JS, Wade PR. Hepatopathy associated with excessive hepatic copper in a Siamese cat. Vet Pathol 1995;32:427–429.
- Meertens NM, Bokhove C a. M, van den Ingh TSG a. M. Copper-associated chronic hepatitis and cirrhosis in a European Shorthair cat. Vet Pathol 2005;42:97–100.
- Asada H, Kojima M, Nagahara T, et al. Hepatic copper accumulation in a young cat with familial variations in the ATP7B gene. J Vet Intern Med 2019;33:874–878.
- Medici V, LaSalle JM. Genetics and epigenetic factors of Wilson disease. Ann Transl Med 2019;7:S58.
- Kim YG, Kim SY, Kim JH, et al. Prevalence and Clinical Relevance of Exon 2 Deletion of COMMD1 in Bedlington Terriers in Korea. J Vet Intern Med 2016;30:1846–1850.
- Stalker MA and Hayes MJ. Liver and biliary system. In Jubb, Kennedy and Palmers Pathology of Domestic Animals. Maxie MG, editor. 5th ed., Elsevier. 2007; pp 297-388.
- Azumi N. Copper and liver injury–experimental studies on the dogs with biliary obstruction and copper loading. Hokkaido Igaku Zasshi 1982;57:331–349.
- Spee B, Arends B, van den Ingh TS, et al. Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs. Journal of veterinary internal medicine 2006;20:1085–92.
- Doong G, Keen CL, Rogers Q, et al. Selected features of copper metabolism in the cat. J Nutr 1983;113:1963–1971.
- Strickland JM, Buchweitz JP, Smedley RC, et al. Hepatic copper concentrations in 546 dogs (1982-2015). J Vet Intern Med 2018;32:1943–1950.
Authored by: T. Stokol and K Miner
