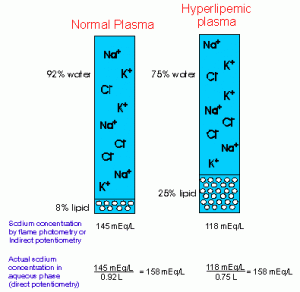
Volume displacement by lipid typically affects electrolyte measurement (principally sodium and chloride, but also potassium), although this is method-dependent. An increased concentration of lipid results in plasma volume with decreased water content and excludes electrolytes (solvent exclusion) into the aqueous portion of plasma. Electrolytes are not found in the lipid portion. Therefore, procedures that measure electrolytes in total plasma volume (per unit of plasma, which includes the lipid fraction), e.g. flame photometry or indirect potentiometry, will result in falsely decreased electrolyte values. This will only occur in very lipemic samples (e.g. triglyceride concentrations >1500 mg/dL). The chemistry analyzer at Cornell University that provides routine electrolyte results on veterinary patients uses indirect potentiometry (in which electrolytes are measured by ion-selective electrodes in diluted plasma). Thus, electrolyte values may be falsely decreased in severely lipemic patients on these chemistry panels. Procedures that measure electrolytes in the aqueous phase only (per unit of plasma water), i.e. direct potentiometry, will result in accurate electrolyte concentrations. Blood-gas analyzers typically use direct potentiometry (i.e. ion-selective electrodes on undiluted plasma), thus this technique is preferred if accurate electrolyte concentrations are desired in patients with severely lipemic plasma.