Critical values
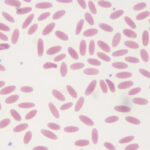
Normal dogs and cats should have platelet counts close to or >200,000/µL; normal horses should have platelet counts of 100,000/µL or greater (foals have higher platelet counts than adults, usually >200,000/µL). Ruminants have higher platelet counts than horses (usually >400,000/µL, particularly in younger animals). Spontaneous bleeding, that can attributed solely to thrombocytopenia, does not occur unless platelet counts are < 30,000/µL, although current guidelines in humans use a cut-off of <10,000/µL (clearly a moving target) for spontaneous bleeds (Jinna and Khandar, accessed February 2021). Studies in mice rendered thrombocytopenic by anti-platelet antibodies show that induced bleeding (tail bleeds) persisted with platelet counts <25,000/µL (Morowski et al 2013). Some animals with platelet counts as low as 10,000/µL have minimal signs of bleeding. Many of these latter animals have large platelets (with increased MPVs, indicating increased megakaryopoiesis), that are thought to be more functional in hemostasis. It is also possible that alterations in vascular integrity from inflammation prompt bleeding in animals with low platelet counts (Goerge et al 2008). There are also guidelines based on when to transfuse human patients with thrombocytopenia (NICE guideline).
Spontaneous hemorrhage should not be ascribed completely to thrombocytopenia at platelet counts of > 30,000/µL. Animals with traumatic injury or surgery may bleed at higher platelet counts (generally <50,000/ul) (Jinna and Khandar). Clinical signs of spontaneous or induced hemorrhage with counts above this value should prompt consideration of additional defects (such as abnormal platelet function or disorders of secondary or tertiary hemostasis) that may be contributing to the hemorrhage . Bleeding due to thrombocytopenia usually is manifested as petechial or ecchymotic hemorrhages in the skin and mucous membranes, retinal hemorrhage, epistaxis, melena, hematochezia, and/or hematuria; essentially, bleeding from small vessels and mucosal surfaces. Severe thrombocytopenia can result in intracavity hemorrhage (hemothorax, hemoabdomen).
Sample considerations
The anticoagulant required for platelet counting is EDTA (purple-top tube). Heparin causes clumping of platelets and is not recommended. In rare situations, an EDTA-dependent pseudothrombocytopenia can occur. This is due to EDTA-induced exposure of platelet antigens, which causes clumping in patients with antibodies (which can be naturally occurring) to these antigens. The result is a low platelet count that is artifactual, but this should be recognized as platelet clumps will be seen in a peripheral blood smear. The only way to get an accurate count in these patients is to collect blood into citrate (blue-top tube) anticoagulant (remembering that the citrate will decrease the platelet count by 10% due to dilution effects; this effect should be compensated for). EDTA-dependent pseudothrombocytopenia has been recognized in horses, but is a rare occurrence. Platelet counts will also decrease with storage (and MPVs increase), therefore counts should be performed as soon as possible after blood collection. Note that clotting of the sample will totally invalidate the platelet count.
Method of measurement
Platelet number can be determined in several ways. Further information on techniques is covered in platelet count under Hematology.
Smear estimate
Assessment of platelet number is part of the routine examination of blood smears; severe thrombocytopenia should be readily recognized by examination of a blood smear. A fairly valid estimate of platelet number can be made from examination of a stained blood film if platelets are distributed singly and randomly throughout the smear. Before estimating platelet counts from a blood smear, the feathered edge of the smear should be examined thoroughly for platelet clumps. Platelet clumps occur readily if there is difficult venipuncture and slow or excessively turbulent blood flow during venipuncture.
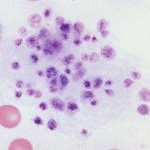
Platelet clumps are more numerous in samples collected from small peripheral veins (which can collapse during venipuncture), rather than larger veins like the cephalic or jugular. The presence of platelet clumps (at the feathered edge or throughout the smear) will decrease a platelet count obtained by any method (estimation, electronic or manual) and may even invalidate the count. Cats have notoriously reactive platelets, which clump readily on sample collection. Therefore, obtaining a platelet count can be difficult in a cat.
Each platelet seen in a microscope field with the 100x oil immersion objective, in the monolayer of the smear, is roughly equivalent to 15,000 platelets/µL. Therefore, if there are more than 10 to 15 platelets per field on average, the platelet count is within or above the reference interval.
Manual count
More accurate quantitation of platelets than a smear estimate can be done manually done by using an Unopette system and counting platelets in a hemacytometer.
Automated counts
Automated hematology analyzers or electronic impedance counters may underestimate the platelet count if platelets are very large (occurs in some cats and dogs, such as Cavalier King Charles Spaniels) or if there are clumps. The platelet count (whether obtained manually or electronically) should always be verified by estimating platelet numbers from a blood smear (with checking for clumps). Warming blood does not minimize platelet clumping or substantially increase platelet clumps in dogs, cats or horses (although the median platelet count increased from a median of 141,000/uL to 196,000/uL in EDTA-anticoagulated blood from 10 horses, as measured with the Sysmex XV-2000IV (Williams and Archer 2016).
Test interpretation
Decreased number (thrombocytopenia)
Pathophysiologic causes of thrombocytopenia fall into one or a combination of the following general mechanisms:
- Decreased production of platelets in the marrow
- Increased consumption or use of platelets in coagulation
- Increased destruction or clearance of platelets by macrophages or the liver
- Sequestration of platelets in the spleen or microvasculature
Increased number (thrombocytosis)
Thrombocytosis may occur in primary myeloproliferative conditions or as a secondary (reactive) phenomenon in a variety of physiologic and pathologic states. Thrombocytosis is usually due to an increase in platelet production and release (in reactive cases, this is likely due to increased thrombopoietin or other thrombogenic cytokines, such as interleukin-6), rather than an increased platelet lifespan. Usually, a platelet count higher than the reference interval for the species is a reactive thrombocytosis, and not of direct pathologic importance. Young animals, in particular calves and foals, normally have platelet counts higher than the adult reference interval.
Further discussion on thrombocytopenia and thrombocytosis is found under disorders of platelet number.
