Most animals with hemostatic disorders present because of signs of excessive hemorrhage and diagnostic testing is most frequently performed in these patients. There are tests for each process of hemostasis (primary, secondary and fibrinolysis) as well as global tests of hemostasis that evaluate two or more of these hemostatic processes simultaneously, as well as the contribution of cells, such as viscoelastic methods or thromboelastography/thromboelastometry (TEG/TEM) and calibrated automated thrombography (CAT) or thrombin generation assays. Global tests require specific and dedicated equipment, which restricts their usage to academic institutions and large specialty practices. Most tests of hemostasis are designed to detect causes of hemorrhage or hypocoagulability and are insensitive to accelerated clotting (hypercoagulability, which predisposes to thrombosis). Some global assays (TEG/TEM) can evaluate fibrinolysis as well as clot formation and have been proported to detect hypo- and hypercoagulability, however additional research studies are required before they can be recommended for detecting hypercoagulable states.
The diagnostic evaluation of an animal with a suspected hemostatic disorder starts with the patient – signalment (age, sex, breed), history, physical examination. Clues obtained from the patient can and should guide diagnostic testing and test interpretation.
Signalment
Inherited disorders typically arise in younger animals and specific breeds, but can occur in any animal, thus should not be discounted if not in a relevant breed. Some inherited disorders are sex-linked and clinical signs are usually only evident in males. In older animals, abnormalities in hemostasis secondary to other disorders (e.g. caner, liver disease) should be suspected first, although bleeding symptoms in animals with mild inherited disorders can manifest at a later age and not when the animals are younger. Animals of any age can ingest anticoagulant rodenticides.
History
Because animals cannot speak for themselves, obtaining a complete history is vital. This should include:
- Episodes of prior hemorrhage: Site (mucosal bleeding, bruises, hematomas), severity, frequency, spontaneous or induced by trauma or surgery (e.g. excessive bleeding noted when chewing a bone). Spontaneous hemorrhage suggests a more severe defect than that induced by trauma or surgery.
- Family history: Symptoms in parents, siblings, progeny?
- Travel history: Exposure to infectious agents that may cause hemostatic defects
- Access to toxicants: This include anticoagulant rodenticides.
- Drug history: Several drugs are known to affect hemostasis (e.g. aspirin, non-steroidal inflammatory drugs, heparin) and should be avoided in animals with inherited bleeding disorders. Any drug can potentially affect hemostasis, so all drugs should be viewed as potential causes for acquired hemostatic abnormalities, until proven otherwise.
Physical examination
Patients that are presented with bleeding should be evaluated for evidence of a local cause of bleeding, e.g. trauma, ulceration, caner, and given a careful physical examination. Routine assessment of heart rate, respiration rate, capillary refill time and oral mucous membrane characteristics is useful for assessment of the overall hemostatic status of the patient. All mucosa (oral, ocular, reproductive) and skin should be examined for evidence of petechiae (pinpoint hemorrhages) and ecchymoses (larger, paintbrush hemorrhages or bruises).
Screening tests
If bleeding symptoms in an animal cannot be attributed to an obvious cause, then a series of screening tests should be done. Hemostasis testing can be done in-house (platelet estimate or count, point-of care coagulation analyzers, ACT, buccal mucosal bleeding time, platelet function analyzers, global hemostasis testing with thromboelastography/elastometry), however most in-house tests are insensitive to mild factor deficiencies or certain defects. Ideally, hemostasis testing should be done by a specialized veterinary laboratory, such as the Comparative Coagulation at Cornell University, where assay performance has been optimized for animal testing. Screening tests should include the following:
- Hemogram, including platelet count: A platelet count is mandatory in any animal presenting with excessive hemorrhage. A complete hemogram is helpful to identify other disorders that may be causing the bleeding symptoms, e.g. inflammation, leukemia.
- Clinical chemistry: To assess for underlying diseases that can affect hemostasis. This is particularly important in an older animal, but should not be discounted in a young animal.
- Screening coagulation assays: The most commonly used screening assays are the prothrombin time (PT) and activated partial thromboplastin time (APTT), with the activated coagulation time (ACT) being available as a less sensitive and more subjective point-of-care test. Some laboratories also include fibrinogen concentration and TCT as screening tests for secondary hemostasis. These tests are usually not specific to an individual defect but assess functioning of a pathway, e.g. intrinsic or extrinsic pathway of coagulation. They are based on trigger reagents, which cause a fibrin clot to form in the sample in the presence of phospholipid and calcium. The clot is then detected through various means (visual, optical, mechanical). Different reagents and methods have their own strengths and weaknesses. Ideally, all clotting assays should be optimized to detect factor deficiencies, but this is really only done by a handful of referral laboratories, particularly those with expertise in hemostasis testing. In particular, testing should be done by a veterinary laboratory because animal plasma clots far quicker than human plasma. Dogs, in particular, have much quicker coagulation times than human beings, and instruments that cannot compensate for these rapid times (many photo-optical instruments) may miss clot formation, yielding erroneous results. In diagnostic laboratories, coagulation assays are frequently incorporated into panels, such as the following:
- Standard panel: PT and APTT. Some laboratories also include fibrinogen measurement and TCT in the standard panel.
- Disseminated intravascular coagulation (DIC) panel: Usually includes PT, APTT, fibrinogen, FDP/D-dimer and antithrombin (AT).
Abnormalities in these screening assays dictate the need for further testing. In some instances, further testing can also include a screening assay (e.g. buccal mucosal bleeding time or BMBT for disorders of primary hemostasis), but usually more specific testing is warranted. Specific testing includes assays for individual factor (e.g. Factor VIII activity for hemophilia A) or inhibitor (e.g. AT activity for DIC) activity or concentration (e.g. vWf antigen testing for von Willebrand disease). In some cases, screening tests are not required and only specific testing is performed, e.g. therapeutic monitoring of heparin, protein C measurement as an ancillary test for portosystemic shunts.
Tests by hemostasis process
The following provides a list of routine and specialized tests of hemostasis by hemostatic process.
- Primary hemostasis: Tests for primary hemostasis involve evaluation of platelet number and function and assessment of von Willebrand factor. Identification of blood vessel wall problems require the appropriate clinical signs (e.g. skin hyperextensibility with fragility in cats with Ehlers danlos syndrome) and histopathologic evaluation of biopsies.
- Platelets
- Platelet count and volume
- Platelet function: Adhesion, release reaction, procoagulant activity, aggregation, genetic defect (breed-specific)
- Global assays (platelet number and function): Buccal mucosal bleeding time (BMBT), platelet function analyzers (PFA), whole blood clotting time, clot retraction
- von Willebrand factor
- Antigen concentration
- Structure (multimers)
- Activity: Collagen binding assay, platelet aggregation (vWf-specific agonists)
- Genetic defect (breed specific)
- Global assays: BMBT, PFA
- Vessel wall: Biopsy
- Platelets
- Secondary hemostasis: Tests for secondary hemostasis involve evaluation of coagulation factors or inhibitors. Some of these assays are quite specialized (e.g. fibrinogen antigen) and only available at select laboratories.
- Coagulation factors
- Screening assays: ACT, PT, APTT, TCT, proteins induced by vitamin K absence/antagonism (PIVKA)
- Specific factor activity: Most factors
- Specific factor concentrations: Fibrinogen antigen
- Genetic defect (factor and breed specific)
- Inhibitors
- Activity: AT, protein C
- Therapeutic heparin monitoring
- Anticoagulant rodenticide screens: High performance liquid chromatography
- Global assays: Viscoelastic methods, thrombin generation assays
- Coagulation factors
- Fibrinolysis: Unfortunately, measurement of many components of the fibrinolytic pathway, including plasminogen, tPA, PAI-1, are not offered routinely, which is a major shortcoming when evaluating animals for hemostatic or thrombotic disorders. The most common tests of fibrinolytic activity are measurement of lytic products, including FDPs and D-dimer.
- Products of degradation: FDPs, D-dimer
- Protein activity or concentration: tPA, plasminogen
- Global assays: Euglobin lysis test, viscoelastic methods using tPA
- Inhibitors: Plasminogen activator inhibitor-1
- Global hemostasis tests: These can measure multiple pathways simultaneously, including the contribution of cells (platelets, erythrocytes):
- Viscoelastic-based methods: Thromboelastography and thromboelastometry
- Calibrated automated thrombography (CAT): Thrombin generation assays
Test interpretation
It cannot be overemphasized that results of hemostasis tests should never be interpreted in isolation; they should always be interpreted in context of the patient (clinical signs, historical details, signalment). Artifactual changes can and do occur, which can yield misleading results. If results do not make sense, then they should be questioned. Treat the patient, not the laboratory data.
There are various ways and substantial controversy on how hemostasis results should be interpreted – with respect to a reference interval or control sample and with respect to the degree of change that is clinically relevant. The main methods used are the following:
- Reference intervals: Usually, results are interpreted with respect to reference intervals established from a healthy group of animals. Abnormal test results can be interpreted as those that fall above or below the limits of the reference interval, regardless of the degree of change (preferred method). This may result in mild abnormalities being over-interpreted (again, look at the patient when interpreting test results). Some investigators only consider a percentage change (e.g. 25%) or absolute change (e.g. 2 seconds) above or below the limit, however this may result in mild defects being ignored or missed. Hemostasis tests do show substantial intra-individual variability or biologic variation (also called low index of individuality, which means large changes can be seen in results obtained from a single animal on different occasions), suggesting that population-based reference intervals may not be the best method of interpretation (this is because they are actually too broad). When this is the case, determination of critical differences may be more appropriate (critical differences are based on biological variation and are applicable to individual animals who essentially serve as their own controls, for example if a critical difference of 3 seconds is determined for a PT assay, this means that the PT results from an individual dog must change by more than 3 seconds from its baseline value to be diagnostically relevant, even if this 3 second increase is within an established reference interval). However, this is usually the default method, because baseline testing is not routinely performed in animals and suitable animals for controls are not always available.
- Control samples: Results are interpreted with respect to a control sample – this could be pooled normal plasma (preferred) or results from an individual healthy animal submitted along with the patient. Again, there are differences in the way that results are interpreted (percentage or absolute change compared to the control). Since most laboratories do not have access to pooled control samples, reference intervals are usually the default method.
Diagnostic testing algorithm
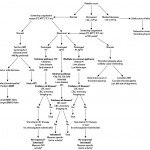
The results of screening tests dictate the need for further or more specific diagnostic testing or may prompt additional assays. The choice of assays (screening or otherwise) should be guided by knowledge of the patient (age, breed, sex, access to anticoagulant rodenticides, parasite exposure, type of bleeding symptoms, presence of underlying disease etc). If assay results are still not fruitful and the animal is still suspected of having a hemostatic disorder, referral of the patient to a hemostasis specialist would be worthwhile for more specialized or complex testing. Referral of the actual patient versus referral of samples collected from the patient is recommended because some testing require the use of fresh platelets from the patient and cannot be done on stored or shipped samples, e.g. platelet function assays. We have provided a suggested diagnostic testing algorithm for a bleeding patient as well as a table of expected test abnormalities with various disorders.
