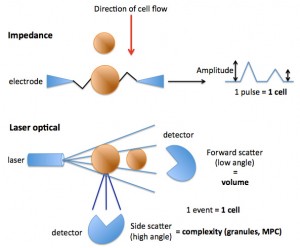
Impedance-based hematologic analyzers allow blood cells, in this case platelets, to pass through an aperture containing an electrical current. As the platelets move through the current, they add resistance and change the amplitude the resistance measurement or pulse. Each pulse is counted as a platelet, whereas the degree of change in amplitude is a measure of the size (volume) of the platelet, with smaller platelets causing less resistance and a lower amplitude.
Laser-based hematologic analyzers allow platelets to pass through in single file through a flow cell containing a laser. As the platelet passes through the laser, it is counted as an event (providing a platelet count) but it also scatters the laser light. Two detectors can measure the scattered light. The first is the forward scatter or low angle light scatter detector, which measures light scattered in a forward direction, which is a reflection of platelet size (more specifically, volume). From the detected laser light, a mean platelet volume (MPV) can be measured and a frequency distribution curve of platelet volume constructed. This is similar to how an impedance analyzer measures and reports on platelet volume, although the latter uses change in amplitude of an electrical current. The second type of detector is a side scatter or high angle light scatter detector, which measured light scattered sideways by the platelet. The degree of light scatter is dependent on the complexity or granularity of the platelet and is reported as a frequency distribution curve and as the mean platelet component (MPC) in g/dL. A smaller platelet will scatter less light in a forward direction, resulting in a lower volume. A less granular platelet will scatter less light in a side direction, resulting in a lower MPC (e.g. activated platelet). This type of measurement cannot be done with an impedance-based analyzer.
Note that with both methods of detection, red blood cells are analyzed similarly but are far larger than platelets (65 FL for a RBC compared to 7 FL for a platelet in a dog) therefore they are counted separately in the same sample by using set volume thresholds. However, in some severely iron deficient animals, particularly camelids, the smaller RBCs can be similar in volume to platelets and are counted incorrectly in the platelet channel. This will falsely lower the RBC count and falsely increase the platelet count. We compensate for this problem (to the best of our ability) by using an impedance-based analyzer with a lowered threshold for counting the smaller RBC in iron deficient camelids, because obtaining an accurate RBC is important in this setting (a falsely low RBC count will increase the MCV, which is a calculated value in camelids, i.e. MCV = PCV/RBC count x 10). It is possible that by decreasing the threshold to detect small RBC, we are also including some platelets in this new RBC count, however this cannot be avoided with current technology.