Plateletcrit
The plateletcrit (reported as a %) is analogous to the hematocrit and reflects the mass of platelets. This result is not usually reported on most hemograms and is infrequently used in laboratories. It has been mainly used to show that dogs with inherited macrothrombocytopenia have normal platelet mass (Tvedten et al 2012, Kelley et al 2014).
Method of measurement
The plateletcrit can be obtained in two ways as indicated below. Results from the two methods do not always correlate, with laser-based method yielding lower plateletcrit than the buffy coat method (Tvedten et al., 2012). At this stage, it is unclear which is the superior method.
- Direct measurement: Buffy coat-based analyzers directly measure the plateletcrit (e.g. VetAutoread™)
- Calculated value: Laser-based optical analyzers calculate a plateletcrit as follows:
Plateletcrit (%) = (MPV x PLT count) ÷ 1000
Test interpretation
A reference interval of 0.129 -0.403% has been established for the plateletcrit (Kelley et al., 2014). Cavalier King Charles Spaniels with inherited macrothrombocytopenia usually (but not always) have a normal plateletcrit, because platelets are uniformly large and their large size makes up for the mild to moderate thrombocytopenia (Tvedten et al., 2012, Kelley et al., 2014). This may explain the lack of bleeding tendencies in these dogs (although platelet counts are rarely below 30 thou/uL), because they have a normal “mass” of platelets. In contrast, Greyhounds can also have lower platelet counts than other breeds of dogs and also have lower plateletcrits. The diagnostic utility of plateletcrit in dogs with acquired causes of thrombocytopenia is currently unknown. It is likely that storage will falsely increase the plateletcrit (platelets swell with storage, increasing the MPV). It is possible that the plateletcrit may help discriminate a true thrombocytopenia and an artifactual thrombocytopenia as a consequence of platelet clumping, however this remains to be tested.
Mean platelet component
This is a measure of the granularity of platelets and can be a reflection of platelet activation. Unactivated platelets contain numerous cytoplasmic granules (alpha granules, dense bodies). When platelets become activated, they degranulate. The granule content of platelets can be assessed using laser optical-based hematologic analyzers reported as the mean platelet component or MPC (in g/dL).
Method of measurement
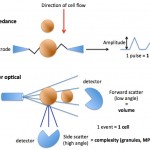
As platelets pass through a laser, they scatter the laser light in a forward and side direction (low and high angle scatter, respectively). Side (high angle) scatter is a measure of the internal complexity of the platelet, which is largely governed by the number of granules and is called the platelet component (PC). Forward (low angle) scatter is a measure of platelet volume. The analyzer provides a mean for both of these values, i.e. mean platelet volume (MPV) and mean platelet component (MPC). A coefficient of variation of the MPC (mean/standard deviation of the PC) or platelet component distribution width (PCDW) is also provided. However, these values are only valid if the platelets are adequately sphered while they pass through the laser (should have a PCDW <5.5 in dogs, Zelmanovic 2006), which is not always the case, regardless of anticoagulant (EDTA is required for adequate sphering). This limits the diagnostic utility of these measurements in animals (since platelets are frequently not sufficiently sphered for analysis). Hence these values are not reported on hemograms and are not used that frequently within the laboratory either.
Test interpretation
If sufficiently sphered, a low MPC indicates that platelets are less granular. This can be due to activation (whether occurring as a real in vivo finding or an artifact of sample collection or storage) or swelling of platelets due to uptake of water (an artifact of storage). Changes in the MPC should correspond to visual features of degranulation in a blood smear (less granular or “gray” platelets). As noted above, the MPC is difficult to interpret if platelets are not adequately sphered (Zelmanovic 2006). A high MPC is of no diagnostic relevance.
Platelet-derived microparticles
Activated platelets, particularly with strong agonists that require uptake of extracellular calcium, such as high concentrations of thrombin, shed membrane-derived microparticles (<1 um; also called microvesicles or extracellular vesicles). These vesicles are rich in phosphatidylserine, increasing the surface area for fibrin formation. They can be quantified in citrated whole blood, platelet-poor plasma (they are buoyant so they do not sediment on standard centrifugation to remove platelets) or platelet-rich plasma, using flow cytometry, although the latter technique has substantial limitations, including ability to resolve such small particles from noise, which depends on the machine used. For quantification, the microparticles are identified by their small size (in relation to spherical beads, which may not show similar scatter properties to platelet microparticles) and binding of both a fluorescent-conjugated annexin V to phosphatidylserine and a different fluorophore-conjugated constitutive platelet-specific marker (to differentiate them from other cell-derived particles), such as CD61 (the β3 integrin chain of the fibrinogen receptor, αIIbβ3 or GPIIb/IIIa). There are limitations with this approach beyond machine size discrimination, including the fact that some platelets may drop off CD61 (e.g. dogs with immune-mediated thrombocytopenia, Dr. Marjory Brooks, personal communication) and not all membrane-derived vesicles express phosphatidylserine. In addition, other cellular derived particles, e.g. released from apoptotic or dying cells, can express phosphatidylserine. Some investigators prefer other lipophilic dyes, such as lactadherin (Dr. Brooks, personal communication), for recognition of these microvesicles. Studies in humans show that of all the cellular-derived microvesicles in blood (endothelial, monocyte, neutrophil, erythrocyte), platelet-derived microparticles (PDMP) are by far the most abundant. Platelet-derived microparticles have been quantified in citrated blood of dogs (Helmond et al 2013), cats (Cremer et al 2018) and horses (Springer et al 2014). Studies in horses show that there are more PDMPs in whole blood than platelet-poor plasma and counts are stable for 24 hours refrigerated or at room temperature (Springer et al 2014). Freezing fractures platelets, increasing microparticle release and causing externalization of phosphatidylserine (Helmond et al 2013). In cats, analytical variation was unacceptably high for platelet-poor plasma but was acceptable for whole blood (Cremer et al 2018). Dogs with Scott syndrome have a defect in TMEM16F (a calcium-dependent scramblase, Brooks et al 2016) and consequently cannot exteriorize phosphatidylserine on platelet membranes or release PDMPs, which allowed recognition of the defect (Brooks et al 2002, Brooks et al 2009). The latter study also showed storage-associated increases in PDMP release and platelet activation (Brooks et al 2009).
