Anaplasma species are intracellular bacteria that are within the family of Anaplasmataceae of the order Rickettsiales. The family of Anaplasmataceae now includes the genera of Ehrlichia, Anaplasma, and Neoricketssia, after renaming of organisms and reclassification in 2001 based on 16S RNA and groESL gene sequences in the bacteria (Allison and Little, 2013). The names of some species, however, remains unchanged, however the genera of Anaplasma now includes pathogenic and non-pathogenic bacteria that infect erythrocytes (e.g. Anaplasma marginale), leukocytes (e.g, Anaplasma phagocytophilum) and platelets (Anaplasma platys).
| Old name | New name | Species affected | Infected blood cell |
| Anaplasma marginale | Anaplasma marginale | Bovine | RBC (extravascular hemolytic anemia) |
| Anaplasma ovis | Anaplasma ovis | Sheep, goats | RBC (non-pathogenic) |
| Ehrlichia equi | Anaplasma phagocytophilum | Horses, dogs, cats, camelids, humans | Neutrophils, eosinophils (pathogenic) |
| Ehlrichia platys | Anaplasma platys | Dogs | Platelets (cyclic thrombocytopenia) |
Bovine
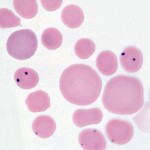
Anaplasma marginale is an intracellular erythroparasite of cattle that produces severe hemolytic anemia and is of major economic importance. Endemic areas in the U.S. are the south and southwest and some parts of California. The disease is transmitted by several species of ticks and is called tick-borne fever. After infection, there is an incubation period of several weeks followed by a period during which parasitemia increases rapidly and is quickly followed by precipitous drop in hematocrit.
The clinical syndrome is one of acute onset, severe anemia with icterus, fever, anorexia, dehydration and depression. Unlike Babesia species, hemoglobinuria does not occur as the hemolysis is extravascular (in the spleen and bone marrow). With acute infection, cattle can die or suffer abortion. Up to 90% of erythrocytes contain the organism. If they survive, several days after the onset of signs, the number of parasites is very low and anemia is regenerative (macrocytic and hypochromic with reticulocytosis). A concurrent immune-mediated hemolytic anemia (with spherocytes) may also be present and results in removal of non-parasitized red cells. Young cattle < 6 months old are resistant to infection. It is acute, and occasionally fatal, in cattle under 3 years of age, whereas older cattle have a 30-50% mortality rate.
Diagnosis of anaplasmosis is confirmed by finding the parasite – it is recognized as small, round dark purple inclusions located at the periphery of red cells. There are usually more than 1-2 per cell. The organism must be differentiated from Howell-Jolly bodies. Serology is also used for diagnosis (IFA, complement fixation, ELISA) as is bacterial DNA detection by polymerase chain reaction (PCR). Effective vaccines have mitigated the economic impact of the disease.
Ovine/Caprine
Anaplasma ovis infects goats and sheep and is usually non-pathogenic (unless the animals are splenectomized).
Equine
- Vector: Ixodes sp (deer ticks).
- Cells: Neutrophils, eosinophils. Morulae are frequently seen in neutrophils, although can degrade rapidly after institution of antimicrobial therapy (losing their characteristic grape-like features).
- Clinical signs: Several syndromes
- Bloodwork: Thrombocytopenia (characteristic), non-regenerative anemia, neutropenia (usually no left shift or toxic change).
- Diagnosis: Morulae in neutrophils in blood. Serologic testing, PCR.
Canine
Two main species of Anaplasma are thought to infect dogs – Anaplasma phagocytophilum (aka Ehrlichia equi) and Anaplasma platys. The former infects granulocytes (neutrophils and eosinophils) whereas the latter infects platelets. Another species of intracellular bacteria that infects dogs and belongs to the family Anaplasmataceae but segregates into a different part of the phylogenetic tree is Ehrlichia ewingii, which like Anaplasma phagocytophilum, infects granulocytes (and is the main differential diagnosis for the latter organism, producing similar disease syndromes). The organisms are transmitted by ticks, particularly Ixodes species, with rodents and deer serving as reservoir hosts (in the northeastern United States, white-footed mice are the implicated main reservoir of not only Anaplasma but also Borrelia burgdorferi). Transmission from the tick to an accidental host, like dogs, is thought to take 36-48 hours (so remove ticks as soon as you see them on yourself or your pets, particularly small brown “deer” ticks, which are Ixodes species). Tetracyclines are the treatment of choice for all Anaplasma infections.
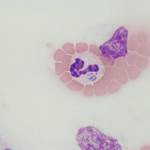
Anaplasma phagocytophilum
- Vector: Ixodes sp (deer ticks).
- Cells: Neutrophils, eosinophils.
- Clinical signs: Fever, lethargy, anorexia are the most common clinical signs and typically occur after 1-2 weeks of infection. Polyarthritis can occur in some infected dogs, resulting in lameness, joint swelling and reluctance to move (Carrade et al 2009). Similarly in horses, common clinical signs are fever, lethargy and “stiff” gaits.
- Bloodwork: A mild to moderate thrombocytopenia is characteristic of infection (around 90% of infected dogs). Leukopenia (mild neutropenia or lymphopenia) and a mild non-regenerative anemia may be seen. Serum biochemical results can show low albumin (likely negative acute phase response) and high globulins (likely due to antigenic stimulation) and increased alkaline phosphatase (may be due to endogenous corticosteroids or “stress”). The mechanisms of these cytopenias is unclear, although the thrombocytopenia could be partly immune-mediated (Carrade et al 2009). Studies have shown that the organism prolongs the circulating lifespan of neutrophils by delaying apoptosis (Borjesson et al 2006). In horses, the most common laboratory finding is a mild thrombocytopenia followed by a mild non-regenerative anemia. Some horses have a concurrent mild neutropenia, which is not usually accompanied by a left shift or toxic change in neutrophils. Some horses have normal laboratory findings. We generally see infections in Fall and Spring, with an increasing frequency of diagnosis in the last few years (associated with global warming and increased tick infestation in Upstate New York).
- Diagnosis: Detection of morulae (colonies of bacteria) in granulocytes (especially neutrophils, but also eosinophils) in blood or synovial fluid. Morulae are not found in all infected dogs (ranges from 36-100%) (Carrade et al 2009) and some dogs only have low numbers of infected granulocytes (0.5 to >30% morulae) (Allison and Little, 2013) so examination of a blood smear cannot be relied upon for diagnosis. The same is true of horses. In dogs, the major differential diagnosis is Ehrlichia ewingii, which induces the same clinical syndromes. PCR (using primers for various specific bacterial genes, such as msp2) can help determine the causative organism, whereas primers targeting species non-specific genetic regions (e.g. 16S rRNA) are not specific for Anaplasma phagocytophilum (Carrade et al 2009). Experimental studies have shown that PCR tests are positive about a week before and for several days after morulae are detected in blood in dogs (Carrade et al 2009). Serologic testing can also be performed however positive titers indicate exposure and not necessarily active disease, since titers can persist for months after infection. Acute and convalescent paired testing can facilitate diagnosis of recent infection, particularly in dogs demonstrating characteristic clinical signs or laboratory features (Carrade et al 2009). Serologic testing also does not reliably differentiate Anaplasma phagocytophilum from other bacterial infections, including Anaplasma platys.
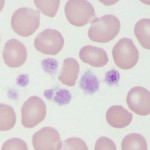
Anaplasma platys
- Vector: Unknown.
- Cells: Platelets. After experimental infection, morulae appear in platelets within 10-14 days, with maximal parasitemia 4 days later. The platelet count drops after this parasitemia and then there is a cyclical parasitemia and thrombocytopenia at 10-14 day intervals (similar to the cycles of anemia seen with Mycoplasma hemofelis infection in cats). The number of parasitized platelets decreases with each cycle, making the diagnosis more difficult over time (Allison and Little, 2013).
- Clinical signs: Fever.
- Bloodwork: Thrombocytopenia (cyclical – see above).
- Diagnosis: Morulae in platelets – must be differentiated from coalesced granules of platelets can strongly mimic morulae, but these should be purple versus blue of the bacteria in a Wright’s stain.
