Synonyms
γ-glutamyl transpeptidase (GGTP)
Physiology
The enzyme γ-glutamyl transferase (GGT) cleaves C-terminal glutamyl groups from amino acids and transfers them to another peptide or to an amino acid. It is important in glutathione metabolism (reduced and oxidized GSH are the main targets), amino acid absorption (cysteine in the kidney), and protection against oxidant injury (Hanigan 2014). GGT is found in many tissues and being membrane-associated, particularly in cells lining organ lumens, its activity is largely restricted to luminal contents. The main source of serum GGT activity is the liver (primarily biliary epithelium), thus GGT is used as a marker of biliary hyperplasia, structural cholestasis (which induces biliary hyperplasia) and biliary necrosis (Bulle et al 1990).
Half life is unknown (3 days is an estimate in horses) (Hoffman).
Organ specificity
GGT is a transmembrane protein and is expressed on the cell membrane, with expression being restricted to the luminal surface of the cell. A small portion (< 5%) is found in the cytoplasm. Solubilization of the membrane with bile acids and increased synthesis result in increased activity in serum, with increased synthesis being secondary to biliary hyperplasia from structural cholestasis (e.g. extrahepatic bile duct obstruction), resulting in increased biliary pressure, or drugs (Bulle et al 1990). The highest concentrations are found in kidney, pancreas, intestine and mammary glands (the latter in dog, cattle, sheep and goats). In one study in calves and horses, the highest activity in calves was seen in the kidney, followed by the pancreas, liver, lung, small intestine and brain, with very little in skeletal or cardiac muscle. In horses, the highest activity was seen in the kidney followed by the liver, pancreas, brain with low activity in other tissues, including mammary gland, skeletal muscle, small intestine and lung (Barakat and Ford 1988). In the liver, it is found primarily on biliary epithelial cells, with small amounts on canalicular surfaces of a few periportal hepatocytes in rats (Bulle et al 1990, Hanigan 2014). Hepatocyte expression of GGT is species-dependent, as GGT is not normally expressed on hepatocytes in mice (Hanigan 2014).
- Biliary tract – source of serum activity in health and disease.
- Pancreas, gastrointestinal tract – GGT does not increase in serum in disorders involving these tissues (it is usually shed into the lumen of these organs).
- Kidney – proximal renal tubules. GGT is shed into urine, rather than blood.
- Mammary glands – GGT is excreted into milk, particularly with colostrum.
- Reproductive tract – Epididymis (GGT is secreted into semen).
Method
Reaction type
End-point
Procedure
GGT catalyzes the transfer of the γ- glutamyl group of L-γ- glutamyl-3-carboxy-4-nitroanilide to glycylglycine to generate L-γ-glutamyl-glycylglycine and 5-amino-2-nitrobenzoate. The amount of 5-amino-2-nitrobenzoate produced is measured photometrically and it is proportional to γ-GT activity. The reaction is shown below:
L-γ-glutamyl-3-carboxy-4-nitroanilide + glycylglycine GGT > 5-amino-2-nitrobenzoate + L-γ-glutamyl-glycylglycine
Units of measurement
Enzyme activity is measured in U/L (U = international unit) and µkat/L (SI units), which is defined as the amount of enzyme that catalyzes the conversion of 1 µmol of substrate per minute under specified conditions. Conversion formula is shown below:
U/L x 0.0167 = µkat/L
Sample considerations
Sample type
Serum, plasma, urine
Anticoagulant
Heparin or EDTA
Stability
The stability of GGT activity in human serum and plasma samples are as follows: 7 days at 15 – 25 °C or 2 – 8 °C, and 1 year at -20 °C.
Interferences
- Lipemia: No significant interference up to 1500 lipemic index.
- Hemolysis: May decrease with moderate to severe hemolysis (≥200 hemolysis index)
- Icterus: Severe icterus may decrease concentrations (>20 mg/dL for unconjugated bilirubin and >50 mg/dL for conjugated bilirubin – the icteric index can be a rough surrogate for these bilirubin concentrations – GGT may be falsely decreased with an icteric index ≥ 20 units).
Test interpretation
Increased GGT activity
In adult animals, this is generally thought to reflect biliary hyperplasia and cholestasis (with bile acids solubilizing GGT off membrane surfaces or cholestasis inducing biliary hyperplasia) (Hoffman). Biliary necrosis can also increase serum activities. Measurement of GGT activity can be a marker of passive transfer of immunity in some species, such as cattle but not horses or camelids. Cancer cells in humans can express GGT, with expression not being restricted to the luminal side of the cell membrane, and GGT may support cancer growth by facilitating amino acid uptake. High serum GGT activity is found mostly in humans with hepatocellular carcinomas but also in those with metastatic liver cancer. Oxidative stress and drugs can stimulate production of GGT in several tissues, such as the lungs and liver, but this may not result in increased GGT activities in serum or plasma (Hanigan 2014).
- Artifact: Underfilling of lithium heparin tubes can increase GGT by 10-50% (some above total allowable error of 20% per ASCVP guidelines), particularly at activities <20 U/L (Lippi et al 2012).
- Drugs: Corticosteroids and phenobarbitone can increase GGT activity in dogs. In one study of dogs given immunosuppressive doses of corticosteroids (4 mg/kg per day for 10 days), the mean GGT activity was increased at day 5 and was attributed to increased synthesis (Solter et al 1994). Anecdotally, we have seen high GGT (and ALP activities) in dogs on corticosteroid therapy. Phenobarbitone is also associated with an increase in GGT activity in dogs (mechanism unclear) (Aitken et al 2003), with mean activities increasing and peaking at 5 and 13-17 weeks after administration of around 5 mg/kg BW for 29 weeks to 12 dogs, however increases were mild with mean results still within the reference intervals (Müller et al 2000).
- Physiologic
- Neonates: Colostrum in most species contains higher GGT concentrations than that in corresponding dam sera. Increases in GGT occur within 24 hours of suckling and are a sensitive indicator of passive transfer in cattle due to very high GGT concentrations in colostrum (10 to 1000 fold in plasma). Very high activity of GGT may be observed (up to 1000 x adult levels in 2-3 day old puppies) that gradually decrease with time (over 10 days in dogs, 2-3 weeks in lambs and calves) and is an expected finding in a neonate (i.e. does not indicate hepatobiliary disease) in calves, crias and dogs and cats (Center et al 1991, Weaver et al 2000, Britti et al 2005, Crawford et al 2006). In Holstein calves, GGT activity can be as high as 3000/uL within 24 hours after birth with adequate passive transfer of immunity but decreases rapidly to be within adult reference intervals by 5-7 weeks of age (Yu et al 2019).The increase in GGT has been used to predict efficacy of passive transfer in calves; in one study, GGT < 200 U/L was 80% sensitive for a diagnosis of failure of passive transfer (Perino et al 1993). In another study, calves with GGT activity below 50 U/L had IgG values consistent with failure of passive transfer of immunity (Parish et al 1997), however this testing should be restricted to young calves (<8 days of age) and measurement of IgG concentrations in serum or plasma is the gold standard and superior to GGT activity. Due to the decrease in GGT activity with age, a GGT activity of 54 U/L was equivalent to passive transfer (IgG > 1000 mg/dL by regression analysis) at 17 days of age (Parish et al 1997). In goats, GGT activity does increase immediately after suckling in kids (Braun et al 1984). Although GGT can be high in crias (average of 130 ± 62 U/L in 48 hour old animals), GGT activities were not correlated to serum IgG concentrations, so it is not considered a good marker of passive transfer of immunity in crias (Weaver et al 2000). Even though foals do not derive GGT from the colostrum of mares, GGT can be higher in neonates (Braun et al 1984, Patterson and Brown 1986). In one study, GGT activity increased transiently (up to 169 U/L) in the 7-28 days after birth, before decreasing to within reference intervals by 2 months of age. Note that increases in ALP activity do occur after suckling. This is thought to be derived from intestinal sources in the neonate and is not due to colostrum itself.
- Breed: Some donkeys and burros can have 2-3 x GGT activity of horses, with a published reference interval (per ASVCP guidelines) of 14-69 U/L (Burden et al 2015), which is higher than our current reference interval for horses but similar to our current reference for donkeys.
- Pathophysiologic: Increases in GGT occur secondary to biliary hyperplasia (increased pressure within the biliary system stimulates hyperplasia) or release of the membrane-associated enzyme secondary to damage to or necrosis of the biliary epithelial cells (Bulle et al 1990), with disruption of intercellular junctions and likely back-leakage of GGT into plasma (Brouillet et al 1998), from toxic compounds or cholestasis (primarily structural versus functional). Solubilization of GGT off hepatocellular membranes may occur with cholestasis from increased bile acid concentrations (e.g. shunting of bile into the systemic circulation of rats increases GGT activity without causing intratubular biliary pressure or structural cholestasis) (Hoffman). Administration of the drug alpha-napththyl isothiocyanate (ANIT) to rats causes biliary necrosis with intrahepatic cholestasis from downregulation of cholangiocyte transporters (Ntcp, OAT) and upregulation of basolateral transporters (e.g. Mrp3), which transport bile acids into the biliary system or blood, respectively. GGT activity increases to peak at 1-2 days after drug administration and then declines, with higher activity of GGT in bile versus serum, suggesting that the increased activity was secondary to necrosis versus cholestasis per se. Intrahepatic cholestasis was seen with increased total bilirubin concentrations and ALP activity (2 days). In contrast, structural cholestasis from extrahepatic bile duct obstruction resulted in higher increases in serum GGT activity, which were first seen at day 1 and peaked at 7 days. Total bilirubin concentrations followed a similar trend in these rats, with ALP activity increased and staying high after day 1. Biliary hyperplasia was observed from days 1-14 and GGT was detected enzymatically in the biliary cells and canalicular surface of periportal hepatocytes. In both ANIT-treated and bile duct-obstructed animals, there was concurrent hepatocellular necrosis with increased AST activity (peaked at 1-2 days) (Bulle et al 1980).
- Hepatobiliary disease:
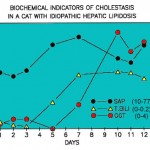
- In small animals, GGT is a sensitive indicator of biliary hyperplasia and structural cholestasis. In one study of 270 dogs with and without liver disaese, the highest increases in GGT activity were seen in steroid hepatopathy, conditions causing intra- and extrahepatic cholestasis (likely structural), and hepatic necrosis. The latter could be due to concurrent biliary necrosis or hyperplasia (Center et al 1992). In the cat, GGT increases may precede increases in ALP and it is considered a more sensitive indicator of liver disease in this species (not in all circumstances; hepatic lipidosis is a notable exception), based on higher numbers of animals with increased GGT (83%) versus ALP activity (43%) (Center et al 1986) (see image above). In one study of 69 cats with and without histologic evidence of liver disease, the highest GGT activities were seen in cats with extrahepatic bile duct obstruction, cholangiohepatitis-cholangitis, and cirrhosis, although numbers of animals in each group were low (Center et al 1986). GGT activity does not increase in small animals treated with carbon tetrachloride (CCl4) toxicity (which causes centrilobular heptocyte necrosis) (Anwer et al 1976). We have seen a few cases of hyperadrenocorticism in dogs, in which there are only increases in GGT activity (with normal ALP activity), which decreases with treatment for the endocrine disorder.
- In large animals, GGT is a sensitive test for biliary hyperplasia (it is a good marker for pyrrolizidine alkaloid toxicity in ruminants) and structural cholestasis (which is relatively uncommon in large animals). Overall, GGT is considered a better marker of biliary tract disorders in large animals than ALP (due to the wide reference intervals in the latter).
- Horses: GGT activity has been reported to be increased in horses with cholangiohepatitis (e.g. secondary to cholelithiasis or proximal enteritis), parvovirus-induced hepatitis (Tomlinson et al 2020) and gastrointestinal problems, including proximal enteritis and colonic displacement (Davis et al 2003, Gardner et al 2005) (high GGT activity may be seen with other gastrointestinal disorders in horses, such as small intestinal strangulating obstruction (Johnstone and Morris 1987)).
- Primary liver disease: With cholangiohepatitis, particularly that due to cholelithiasis, a higher fold increase in GGT activity as compared to AST and ALP activities can be a useful diagnostic feature (Peek and Divers 2000). Monitoring of GGT activity is useful in assessing response to antibiotic therapy in this disorder. GGT activity has been shown to increase with 1 day of treatment of 3 horses with chlorof0rm and remained high for 16 days (Barakat and Ford 1988). However, it is not clear if this is truly due to necrosis (of biliary epithelium or hepatocytes) or secondary to localized cholestasis (such as from hepatocyte swelling). The sustained increases in GGT activity in the latter study likely reflect biliary hyperplasia. In horses with experimental equine parvovirus infection, increases in GGT activity can occur with increases in SDH and GLDH activities, but usually lags by several days to up to 2 weeks. The peak activities ranged from 81-233 U/L in the individual horses. Increased GGT activity can persist for a longer duration (2-6 weeks) after normalization of SDH and GLDH activities. The cause of the high GGT activity was unclear, but a mild increase in direct bilirubin was seen in a few horses; biliary necrosis and hyperplasia may be at play (Tomlinson et al 2020). Hence, a high GGT activity in isolation should raise the possibility of equine parvovirus infection (the likely cause of Theiler’s disease or equine serum hepatitis). Several racehorses with high GGT syndrome (increases in GGT activity associated with training) do have high SDH activity, supporting liver injury in a subset of horses (Ramsay et al 2019), but there are no identified links between high GGT syndrome and equine hepatotropic viruses, including parvovirus (Ramsay et al 2019) and hepacivirus (internal studies).
- Gastrointestinal issues: In the Gardner 2005 study, 48% and 2% of 37 and 45 horses with right and left colonic displacement, respectively, had GGT activities above the reference interval (as high as 489 and 92 U/L, respectively). Although the mechanism of the GGT increase is not always clear in an individual horses, some horses with colonic displacement and cholangiohepatitis do have biochemical (increased direct bilirubin in serum) and/or histologic evidence of biliary hyperplasia or cholestasis (Davis et al 2003, Gardner et al 2005). Cholestasis with right colonic displacement could be due to stretching and direct compression of the bile duct by the displaced organ (Gardner et al 2005), whereas cholestasis with proximal enteritis could be secondary to cell swelling from hepatic injury, which may be in response to an ascending bacterial infection from the intestine or absorption of bacterial toxins via the portal circulation. Vacuolar change is seen in hepatocytes and biliary epithelial cells in this disorder and some animals have a mixed lymphoplasmacytic or suppurative cholangiohepatitis on histopathologic evaluation of the liver. A functional cholestasis (inflammatory cytokine downregulation of bile or bilirubin transporters) may also be operative in some animals.
- High GGT syndrome in racehorses: An increase in GGT activity is seen in racehorses with training and activities > 100 U/L are associated with poor performance (Ramsay et al 2019). The cause of the increase in GGT activity is unknown, but is likely multifactorial, including hypoxic liver injury, oxidative stress and anti-oxidant depletion, and possibly cholestasis (Mann et al 2021).
- Ruminants: In one study of hepatic injury in ruminants, there were no consistent changes in GGT activity after administration of chloroform in 4 calves, whereas GLDH and SDH activities increased (Barakat and Ford 1988). A mild increase in GGT activity was seen in the latter study in 1 of two sheep 1 day after administration of chloroform with the increases being sustained for 16 days. However, since the number of tested animals were low in the latter study and no control was administered, these results should be interpreted with caution.
- Horses: GGT activity has been reported to be increased in horses with cholangiohepatitis (e.g. secondary to cholelithiasis or proximal enteritis), parvovirus-induced hepatitis (Tomlinson et al 2020) and gastrointestinal problems, including proximal enteritis and colonic displacement (Davis et al 2003, Gardner et al 2005) (high GGT activity may be seen with other gastrointestinal disorders in horses, such as small intestinal strangulating obstruction (Johnstone and Morris 1987)).
- Hepatocytes can express low amounts of GGT on their canalicular surface in human adult livers (Irie et al 2007) and fetal hepatocytes express GGT [Shiozawa et al 1990]. Liver disease (viral disease, alcoholic hepatitis and cancer, such as hepatocellular carcinoma) can be associated with high GGT activity in humans (Irie et al 2007, Hanigan 2014), so increases in serum GGT activity can reflect biliary (primarily) or, rarely, hepatocellular disease.
- Renal disease: GGT is expressed on the membranes of proximal renal tubular epithelial cells. Cell injury causes GGT to be shed into the urine and not into blood. The urinary GGT to creatinine ratio has been studied as an early indicator of renal tubular injury, especially due to aminoglycoside toxicity (Garry et al 1990, Grauer et al 1995).
- Hyperadrenocorticism: A few dogs with Cushing’s syndrome may have increased GGT, but not ALP, activity, which tracks with resolution and recurrence of the disease after mitotane therapy (personal observations, Cornell University). The reason for these discrepant or unexpected changes is unknown.
- Hepatobiliary disease:
ALP/GGT changes
- In cats with hepatic lipidosis, ALP activity will be increased early in the progression of the disease, followed by an increase in bilirubin concentrations (combination of direct and indirect) and finally in GGT activity (see image to the right). Thus, increases in ALT, AST and ALP activities with hyperbilirubinemia is often indicative (but not 100% specific for) lipidosis.
- Cats can show higher increases in GGT over ALP activity in cases of necroinflammatory disorders involving the biliary structures or pancreas.
- Cats with hyperthyroidism may show mild increases in ALP, AST and ALT activities, whereas GGT activity and total bilirubin concentrations are normal.
- GGT may be increased to a greater degree than ALP activity in diseases associated with biliary hyperplasia in the absence of cholestasis (eg biliary carcinoma, pyrrolizidine toxicosis or Fasciola infections in cattle).
- Sustained increases in ALP and GGT activity have been recognized to occur during the healing phase following hepatic necrosis (Barakat and Ford 1988).
References
- Hoffman WE and Solter PF. Diagnostic enzymology of domestic animals. In: Clinical Biochemistry of Domestic Animals, eds. Kaneko JJ, Harvey JW and Bruss M. 6th edition. Academic Press. 2008; pp:351-378. https://doi.org/10.1016/B978-0-12-370491-7.X0001-3
