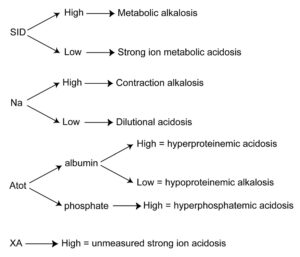
Flow chart for interpreting acid-base results from a chemistry panel based on strong ion principles. In the strong ion approach to acid-base status, pH, partial pressure of carbon dioxide (respiratory component) and strong ions (metabolic component) are independent variables, whereas bicarbonate is a dependent variable. Strong ions include sodium (cation), chloride (anion), phosphate (anion), protein (particularly albumin; considered an anion) and unmeasured ions (XA). Cations are thought of as bases and anions as acids (because the latter can bind hydrogen), therefore increases in cations (high sodium) causes an alkalosis whereas increases in anions (chloride, phosphate, proteins (albumin), unmeasured anions) causes an acidosis. Similarly, decreased cations (sodium) causes an acidosis and decreased anions (proteins, primarily albumin) causes an alkalosis.
The strong ion difference (SID) in this chart is calculated as the sum of sodium and potassium – chloride, i.e. SID = (sodium+potassium)-chloride