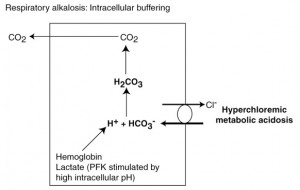
In a primary respiratory alkalosis, pCO2 is decreased, resulting in a higher pH in blood and within cells than normal. To offset this, intracellular buffers (mostly hemoglobin within red blood cells) release hydrogen. Hydrogen combines with bicarbonate (moves into the cell from plasma in exchange for chloride) to form carbonic acid, which then disassociates to form water and CO2, thus replenishing the depleted pCO2 and removing bicarbonate (a base) from blood, thus offsetting the increase in pH. The high intracellular pH also generates lactic acid intracellularly (phosphofructokinase is stimulated by high intracellular pH), which also liberates its hydrogen to help drive the reaction. A compensatory hyperchloremic metabolic acidosis also occurs in response to a primary respiratory alkalosis through the kidney decreasing ammonium chloride excretion in the proximal renal tubules (however this does not occur immediately, but takes hours to day to have any effect).