The body strives to maintain electroneutrality at all times by keeping the concentrations of anions equivalent to cations in serum (or plasma). In a healthy individual, sodium and potassium (also called “measured” cations) account for about 95% of total cations, whereas chloride and bicarbonate (also called “measured” anions) account for about 85% of the total anions. “Unmeasured” cations (UC+) and anions (UA–, see below) account for the differences. This concept of electroneutrality can also be demonstrated graphically in a gamblegram.
Concept of electroneutrality
Sum of cations = Sum of anions
or
(Na+ + K+ + UC+) = (Cl– + HCO3– + UA–)
The anion gap reflects the difference in the serum (plasma) concentrations of the “measured” cations and “measured” anions and is calculated using the following formula:
Anion gap = (Na+ + K+) – (Cl– + HCO3–)
However, we need to incorporate “unmeasured” cations and “unmeasured” anions in the above equation, as follows:
Anion gap = (Na+ + K+ + UC+) – (Cl–+ HCO3– + UA–)
or (rearranging the above equation)
Anion gap = UA– – UC+ = (Na+ + K+) – (Cl– + HCO3–)
or
Anion gap = UA– – UC+
Thus, the anion gap is calculated using “measured” cations (sodium, potassium) and anions (chloride and bicarbonate) in mEq/L, but actually is an indicator of the difference between “unmeasured” anions and cations. Since “unmeasured” anions are more abundant than unmeasured cations and are more important in terms of disease than “unmeasured” cations, the anion gap is essentially a marker of the amount of “unmeasured” anions in circulation.
“Unmeasured” anions (UA–)
These include proteins that are normally negatively charged at physiologic pH (particularly albumin, which is the largest contributor to the anion gap in health) and noncarbonic acids that are produced during physiologic and pathologic processes, including lactate, phosphates, sulfates, and ketones. Some exogenous toxins and drugs, including methanol, salicylate and ethylene glycol (and its metabolites), are also “unmeasured” anions. Because “unmeasured” anions are found in higher concentrations than “unmeasured” cations, they have a far greater impact on the anion gap.
Note that the term “unmeasured” is really a misnomer, as many of these anions and cations are actually or can be measured, however they are not included in the anion gap equation (and are “unmeasured” for this purpose).
“Unmeasured” cations (UC+)
These include proteins that are positively charged at physiologic pH (γ-globulins) and the free or ionized forms of calcium (Ca2+) and magnesium (Mg2+). The latter are not seen in high enough concentrations to make much impact on the anion gap.
Use of anion gap for determining causes of metabolic acidosis
Changes in anion gap are used primarily to distinguish between causes of a metabolic acidosis, i.e. due to titration of bicarbonate or loss of bicarbonate.
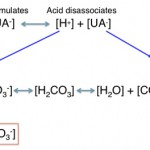
- Metabolic acidosis due to titration: There will be accumulation of a noncarbonic acid, whose proton (H+) is being buffered by bicarbonate (HCO3–), leading to a decrease in bicarbonate and an increase in the anion of the acid. The anion of the acid is an “unmeasured” anion (UA–) and will thus increase the anion gap. This concept is illustrated in the image to the right.
- Acidosis due to bicarbonate loss or changes in strong ions: With bicarbonate loss or loss of sodium in excess of chloride, there is a gain of chloride-containing acid, thus the anion gap is normal in a bicarbonate loss acidosis but there is a decreased strong ion difference ([Na+ + K+]-Cl–) and the chloride is disproportionally higher than the sodium.
The table below summarizes the changes seen in the anion gap in these two forms of metabolic acidosis:
| Mechanism of low bicarbonate | Expected anion gap | Strong ion difference (Na+ + K+)-Cl– |
Chloride vs sodium |
| Titration by excess noncarbonic acid | Increased | Normal | The same |
| Bicarbonate or sodium loss, gain of a chloride-containing acid | Normal | Decreased | High |
Method of measurement
Refer to individual anions/cations homepage ( Na+, K+, Cl–, and HCO3–)
Sample considerations
Refer to individual anions/cations homepage ( Na+, K+, Cl–, and HCO3–)
Test interpretation
Increased anion gap
This indicates increased unmeasured anions (more common) or decreased unmeasured cations (less common).
- Artifact: Any artifactual cause of increased sodium or potassium or decreased chloride or bicarbonate (e.g. storage of blood on cells with lactate production by cells, which consumes bicarbonate in the tube) may increase the anion gap. This is an uncommon cause of a high anion gap.
- Iatrogenic: Administration of sodium-containing drugs such as sodium salts and penicillin may increase the anion gap because their sodium content is measured as part of the sodium concentration on a chemistry panel.
- Pathophysiologic:
- Titration or high anion gap metabolic acidosis: This is the most common cause of an increased anion gap and is due to accumulation of an noncarbonic (nonvolatile) acid, such as L- or D-lactate, ketones and uremic acids (e.g. sulfates, phosphates). Some toxins and drugs are also acids (methanol, salicylate, ethylene glycol metabolites) and toxicity with these compounds is characterized by a high anion gap (or titration) metabolic acidosis. This type of acidosis is almost always a primary metabolic acid-base disturbance. In one study of neonatal foals (within one week of age) with various disorders (sepsis, neonatal encephalopathy, enterocolitis etc), an anion gap of >27 mEq/L had a sensitivity of 80% and specificity of 85% for detecting a high L-lactate (> 7 mmol/L) in a subsample of 81 foals. However, the change in the anion gap explained only about 46% of the change in L-lactate by linear regression analysis, indicating other unmeasured anions in these sick foals contribute to the anion gap (Gomez et al 2015).
- Alkalemia: Loss of protons (H+) from plasma proteins (particularly albumin) in an attempt to buffer the increase in bicarbonate, increases their net negative charge. Alkalemia also stimulates lactic acid production (a high intracellular pH stimulates phosphofructokinase in cells which drives anaerobic metabolism). The lactate may move into plasma (from within cells), which can increase the anion gap. The increase in AG in both situations is usually mild (<3 mEq/L, Dhondup and Qian 2017) and the L-lactate production likely dominates as the cause of a mildly increased anion gap in an animal with alkalemia and without evidence of a cause for a concurrent high anion gap acidosis (which is common in such animals).
- Increased albumin: This may increase the anion gap because it is an “unmeasured” anion, e.g. dehydration, increased albumin production This will have a mild effect on anion gap.
- Decreased “unmeasured” cations: These have minimal affect on the anion gap because of their low concentrations normally (free ionized calcium, free ionized magnesium).
Decreased anion gap
This is usually a laboratory artifact, with measurement of the electrolytes that make up the anion gap (sodium, potassium, chloride, bicarbonate). Other causes include decreased unmeasured anions or increased unmeasured cations.
- Artifact: A falsely high chloride or bicarbonate measurement will decrease the anion gap. The anticonvulsant drug, bromide, is measured as “chloride” by ion-selective electrodes and will falsely increase chloride concentrations and thus decrease the anion gap. We also have seen this artifact with zonisamide therapy for seizures. Similarly, very high concentrations of pyruvate and lactate dehydrogenase (both of which can interfere with the enzymatic reaction used for bicarbonate measurement) secondary to severe muscle injury may falsely increase bicarbonate concentrations and decrease the anion gap, but this is quite rare.
- Iatrogenic: Fluids containing high concentrations of bicarbonate will decrease the anion gap, by increasing bicarbonate values on the chemistry panel. This rarely happens with chloride-rich fluids since the chloride is administered with sodium (NaCl).
- Pathophysiologic:
- Decreased albumin: This may result in a lower than expected or (rarely) a decreased anion gap. Hypoalbuminemia can be due to various reasons, e.g. hemodilution. A decrease in albumin of 1 g/dL may decrease the anion gap by 2.5-3 mEq/L (Dhondup and Qian 2017).
- Administration of bicarbonate-rich fluids.
- Increased “unmeasured” cations: Since free ionized magnesium and free ionized calcium concentrations are low (compared to other positively charged compounds) and very marked increases are not compatible with life, increases in these two “unmeasured” cations will generally have a mild impact on the anion gap (if any impact at all). Marked increases in γ-globulins (e.g. with B cell or plasma cell neoplasia, such as multiple myeloma) could potentially decrease the anion gap assuming these proteins retain their slightly cationic charge (γ-globulins are normally cationic at physiologic pH), however we do not see this effect on the anion gap frequently with monoclonal gammopathies.
- Acidemia: Protons released from accumulated acids are buffered by plasma proteins (e.g. albumin), which decreases their normal negative charge. This occurs minimally because most accumulated acids are noncarbonic acids and are buffered by bicarbonate and these acids are unmeasured anions which will have a greater effect on the anion gap (increasing it), then the acidemia itself decreasing the negative charge on protein buffers (see above for decreased albumin). It is possible that a decreased anion gap in an acidemia due to a hyperchloremic metabolic acidosis (bicarbonate loss, which typically has a normal anion gap) could be due to this mechanism, but in reality a low anion gap is hardly ever seen in a hyperchloremic metabolic acidosis.
Normal anion gap
The anion gap will be normal when there are no abnormalities in the animal, but may also be normal in a strong ion metabolic acidosis (see above).
