Interpretation
Mass at thoracic inlet: Endocrine tumor, likely parathyroid in origin, with concurrent hemorrhage
Explanation
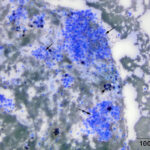
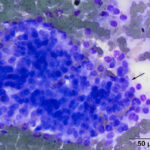
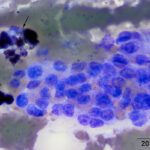
The aspirate contained many dense circular clusters (nests or packets) of uniform cuboidal to low columnar epithelial cells, with rare acinar-like arrangements, in a background containing large amounts of blood. The cells had a moderate amount of light blue slightly grainy cytoplasm with basilar nuclei containing lightly clumped chromatin and no visible nucleoli. Anisokaryosis and anisocytosis were mild. There were also moderate numbers of macrophages, which were erythrophagocytic or contained hemosiderin in their cytoplasm or both (Figures 2-4). The features of the cells are characteristic of a hormone-producing epithelial tumor (e.g. parathyroid, thyroid cytology question 1). With the marked hypercalcemia and hypophosphatemia, a parathyroid tumor of chief cell origin (adenoma or well-differentiated carcinoma) was prioritized (cytology question 2).
 |
 |
 |
The most noteworthy results on the serum biochemical panel were a marked hypercalcemia and hypophosphatemia. This pattern of changes is characteristic of excess PTH from primary hyperparathyroidism1 or chronic kidney disease.2 Although the horse had an increased creatinine concentration with isosthenuric urine, indicating a renal azotemia, the mild nature of the renal disease (based on the degree of increase in the creatinine concentration) did not explain the marked changes in calcium and phosphate concentrations, prioritizing primary hyperparathyroidism as the diagnosis. Other than excess PTH from primary hyperparathyroidism, PTHrP secretion from a non-parathyroid neoplasm producing a paraneoplastic hypercalcemia would be the second main differential diagnosis (i.e. before chronic kidney disease) but is ranked lower than primary hyperparathyroidism because high PTHrP concentrations usually causes hypercalcemia without hypophosphatemia (but can absolutely do both) (chemistry question 1). To differentiate between these causes, imaging studies to look for an underlying parathyroid lesion or tumor producing PTHrP, such as gastric squamous cell carcinoma,3 and a hypercalcemia malignancy panel should be performed as done in this horse (chemistry question 2). Results of the malignancy panel showed a marked increase in free ionized calcium (3.63 mmol/L; reference interval [RI], 1.58-1.90 mmol/L) and PTH (30.8 pmol/L, RI, 0.6-11 pmol/L) and a PTHrP concentration of 0 pmol/L, confirming the presumptive diagnosis of primary hyperparathyroidism.
The hyperglycemia could have been a transient stress-related response (increased epinephrine or endogenous glucocorticoids), however insulin resistance from the diagnosed PPID could also be operative. The increased total and indirect bilirubin concentrations were attributed to the inappetence. The high LDH activity with no changes in liver enzyme (SDH, GLDH, AST) or muscle (CK, AST) activities is an unusual finding and may be secondary to the neoplasm. High LDH activity has been identified as a tumor marker in humans and dogs,4,5 but not horses. Note that the hypercalcemia may be contributing to the isosthenuric urine by increasing medullary blood flow (resulting in medullary solute washout) and inhibiting the action of ADH in the collecting ducts (via decreasing aquaporin2).
Outcome and additional tests
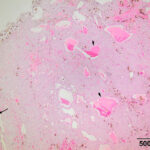
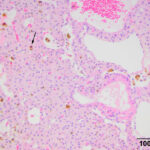
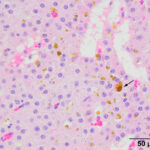
The mass at the thoracic inlet was surgically excised, fixed in formalin, and submitted for histologic evaluation. Histologic evaluation of hematoxylin-&-eosin-stained slides of the paraffin-embedded fixed tissue showed an expansile mass consisting of medium p0lygonal cells forming trabeculae, cords, and packets, with rare microfollicular arrangements. The cells were supported by a thin fibrovascular stroma. Areas of hemorrhage with hemosiderin-containing macrophages were seen in the stroma, along with dilated blood vessels (Figure 5-7). The tumor cells had large amounts of eosinophilic cytoplasm with round nuclei and displayed mild anisokaryosis and anisocytosis, with 1 mitotic figure in 10 2.37 mm fields. The histologic diagnosis was a parathyroid adenoma. The free ionized calcium concentration decreased to within the reference interval (1.66 mmol/L) 6 days after surgery. The horse was then lost to follow-up.
 |
 |
 |
Discussion
Parathyroid disorders are uncommon in the horse, with reports of primary and secondary hyperparathyroidism1,6–8 and hypoparathyroidism.9,10 In a retrospective study of 17 cases of primary hyperparathyroidism in horses,1 affected animals were mostly older (median, 21 years, range, 10-34 years) and of various breeds, including Quarter Horse, Morgan, Warmblood, mule and pony. The most frequent clinical signs were weight loss, anorexia and lethargy, as seen in the case herein. Of the 17 horses, three horses were presented due to a documented hypercalcemia on blood testing.1 Affected horses had a total (median, 17.3 mg/dL; range; 14.6-26.6 mg/dL; 17/17) and ionized (median, 2.66 mmol/L; range, 2.14-4.95 mmol/L; RI, 1.44-1.74 mmol/L; 15/15) hypercalcemia on chemistry testing and 71% were hypophosphatemic (range, 0.9-1.9 mg/dL; RI, 2.1-4.7 mg/dL). PTH concentrations were increased in 71% (11.7-119 pmoL/L) or within reference intervals for the remaining horses (3.9-6.7 pmoL/L; RI, 0.6-11.0 pmoL/L). However, a normal PTH concentration is inappropriate in an animal with a high free ionized calcium concentration and still supportive of a diagnosis of hyperparathyroidism. Masses, usually single, were identified with ultrasonography or scintigraphy in 12/14 horses in the thoracic inlet, mid-neck or around the thyroid region. Two horses had multiple masses. As in this case, surgical treatment was elected in 10 horses with single masses and the hypercalcemia normalized after surgery in 5/6 cases with a confirmed histologic diagnosis of parathyroid adenoma. In the remaining 4 surgical cases, histologic results on the removed tissue were reported as normal tissue for the region (thyroid, parathyroid, connective tissue) with one thyroid adenoma (one could speculate this was a parathyroid and not thyroid adenoma, given the hypercalcemia) and one branchial cyst. Common post-operative complications included hypocalcemia, ongoing or new hypophosphatemia, and kidney injury.
Hypercalcemia is an uncommon abnormality in chemistry panels from horses. In small animals, the acryonym of HARDIONS is used to help people remember differential diagnoses for hypercalcemia – H: Hyperparathyroidism, A: Addison’s disease, R: Renal disease, D: Vitamin D toxicosis/excess, I: Idiopathic, O: Osteolytic lesions, N: Neoplasia, and S: Spurious. However, of these conditions, renal disease would be the top differential diagnosis for a horse with hypercalcemia and hypophosphatemia,2,11 followed by hyperparathyroidism, and hypercalcemia of malignancy, with more rare disorders, such as vitamin D toxicosis (e.g. ingestion of vitamin D-containing plants like Cestrum)12 and idiopathic granulomatous disease13 (the latter may be due to excess vitamin D production14), being further down the list. Various neoplasms have been associated with paraneoplastic hypercalcemia in horses, including gastric squamous cell carcinomas3 and multiple myeloma15,16 with case reports or small case series of a vulvar squamous cell carcinoma,17 ameloblastoma,18 lymphoma,19 a mesenchymal tumor of presumptive ovarian origin,20 and other carcinomas.21,22 With these neoplasms, paraneoplastic production of PTHrP is thought to be the main mechanism for the hypercalcemia,16,18 although vitamin D production could be contributing as well. Hypercalcemia and hypophosphatemia is more commonly seen in horses with chronic renal disease2 but can be observed in animals with acute kidney injury.23 These biochemical changes are attributed to the major role of the kidney in excreting the large amounts of calcium normally ingested by horses in their diet. The mechanism for hypophosphatemia in equine renal disease is less clear, but may be due to failure of proximal renal tubular absorption of filtered phosphate or secretion of phosphatonins. With hyperparathyroidism, PTH from the chief cells induces hypercalcemia via promoting osteolysis (via PTH stimulating osteoblasts to secrete factors which stimulate osteoclastogenesis and osteoclast activation) and resorption of calcium in the loop of Henle, distal tubules and collecting duct of the kidney. PTH also promotes the conversion of vitamin D to its active form in the kidney via activating the enzyme 1-α hydroxylase (the latter does not occur in horses, because they lack 1-α hydroxylase). The hypophosphatemia is due to PTH-mediated inhibition of proximal renal tubular resorption of filtered phosphate. Despite the marked hypercalcemia, horses with primary hyperparathyroidism do not appear to have concurrent renal disease (perhaps because of the concurrent hypophosphatemia). It is likely in the horse of this report that the chronic kidney disease was a comorbidity versus a consequence of the hypercalcemia.
The cytologic features of the cells in the smears of the aspirate of the thoracic inlet mass in this horse were classic for an endocrine (or neuroendocrine) tumor, i.e. the cells were found in three-dimensional “packets” or nests, they were of medium size and uniform in appearance, and had indistinct boundaries. Another feature of aspirates from these tumors is “bare” or “naked” nuclei in a background of cytoplasm, because the epithelial cells tend to be fragile and rupture readily during smear preparation. However, naked nuclei are not invariably seen in smears of aspirates of these tumors, as illustrated in this case. Distinction between a parathyroid adenoma or carcinoma requires histologic evaluation of tissue architecture, assessing for vascular and tissue invasion and high mitotic activity, features that were lacking on histologic evaluation of this case. The location of the tumor in the thoracic inlet is aberrant but thyroid and parathyroid tissue can occur in ectopic locations from the usual location in the ventral upper to middle region of the neck to the mediastinum.24
Author: T Stokol
Acknowledgements: Dr. Tran read out the histology report and Dr. Todd-Donato provided the scintigraphic image.
References
- Gorenberg EB, Johnson AL, Magdesian KG, Bertin FR, Costa LRR, Theelen MJP, et al. Diagnosis and treatment of confirmed and suspected primary hyperparathyroidism in equids: 17 cases (1999-2016). Equine Vet J. 2020 Jan;52(1):83–90.
- Tennant B, Bettleheim P, Kaneko JJ. Paradoxic hypercalcemia and hypophosphatemia associated with chronic renal failure in horses. J Am Vet Med Assoc. 1982 Mar 15;180(6):630–4.
- Taylor SD, Haldorson GJ, Vaughan B, Pusterla N. Gastric neoplasia in horses. J Vet Intern Med. 2009 Oct;23:1097–102.
- Khan AA, Allemailem KS, Alhumaydhi FA, Gowder SJT, Rahmani AH. The Biochemical and Clinical Perspectives of Lactate Dehydrogenase: An Enzyme of Active Metabolism. Endocr Metab Immune Disord Drug Targets. 2020;20(6):855–68.
- Marconato L, Crispino G, Finotello R, Mazzotti S, Salerni F, Zini E. Serum lactate dehydrogenase activity in canine malignancies. Vet Comp Oncol. 2009 Dec;7(4):236–43.
- McKenzie RA, Gartner RJ, Blaney BJ, Glanville RJ. Control of nutritional secondary hyperparathyroidism in grazing horses with calcium plus phosphorus supplementation. Aust Vet J. 1981 Dec;57(12):554–7.
- Ronen N, van Heerden J, van Amstel SR. Clinical and biochemistry findings, and parathyroid hormone concentrations in three horses with secondary hyperparathyroidism. J S Afr Vet Assoc. 1992 Sep;63(3):134–6.
- Colmer SF, Wulster K, Johnson AL, Levine DG, Underwood C, Watkins TW, et al. Treatment of primary hyperparathyroidism in a Miniature Horse using chemical ablation of abnormal parathyroid tissue localized by 3-phase computed tomography. J Vet Intern Med. 2022 Mar;36(2):798–804.
- Rivas VN, Magdesian KG, Fagan S, Slovis NM, Luethy D, Javsicas LH, et al. A nonsense variant in Rap Guanine Nucleotide Exchange Factor 5 (RAPGEF5) is associated with equine familial isolated hypoparathyroidism in Thoroughbred foals. PLoS Genet. 2020 Sep;16(9):e1009028.
- Thompson AC, Mochal-King C. Primary Hypoparathyroidism and Recurring Hypocalcemia in a Quarter Horse Gelding-A Case Report. J Equine Vet Sci. 2021 Apr;99:103398.
- Leroy B, Woolums A, Wass J, Davis E, Gold J, Foreman JH, et al. The Relationship between Serum Calcium Concentration and Outcome in Horses with Renal Failure Presented to Referral Hospitals. J Vet Intern Med. 2011 Nov;25:1426–30.
- Krook L, Wasserman RH, Shively JN, Tashjian AH, Brokken TD, Morton JF. Hypercalcemia and calcinosis in Florida horses: implication of the shrub, Cestrum diurnum, as the causative agent. Cornell Vet. 1975 Jan;65(1):26–56.
- Sellers RS, Toribio RE, Blomme EA. Idiopathic systemic granulomatous disease and macrophage expression of PTHrP in a miniature pony. J Comp Pathol. 2001;125(2–3):214–8.
- Tebben PJ, Singh RJ, Kumar R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr Rev. 2016 Oct;37(5):521–47.
- Edwards DF, Parker JW, Wilkinson JE, Helman RG. Plasma cell myeloma in the horse. A case report and literature review. J Vet Intern Med. 1993;7(3):169–76.
- Barton MH, Sharma P, LeRoy BE, Howerth EW. Hypercalcemia and high serum parathyroid hormone-related protein concentration in a horse with multiple myeloma. J Am Vet Med Assoc. 2004 Aug 1;225:409–13, 376.
- Karcher LF, Le Net JL, Turner BF, Reimers TJ, Tennant BC. Pseudohyperparathyroidism in a mare associated with squamous cell carcinoma of the vulva. Cornell Vet. 1990 Apr;80(2):153–62.
- Rosol TJ, Nagode LA, Robertson JT, Leeth BD, Steinmeyer CL, Allen CM. Humoral hypercalcemia of malignancy associated with ameloblastoma in a horse. J Am Vet Med Assoc. 1994 Jun 15;204(12):1930–3.
- Mair TS, Yeo SP, Lucke VM. Hypercalcaemia and soft tissue mineralisation associated with lymphosarcoma in two horses. Vet Rec. 1990 Feb 3;126(5):99–101.
- McCoy DJ, Beasley R. Hypercalcemia associated with malignancy in a horse. J Am Vet Med Assoc. 1986 Jul 1;189(1):87–9.
- Cook G, Divers TJ, Rowland PH. Hypercalcemia and erythrocytosis in a mare associated with a metastatic carcinoma. EqVetJ. 1995;27:316–8.
- Fix AS, Miller LD. Equine adrenocortical carcinoma with hypercalcemia. Vet Pathol. 1987 Mar;24(2):190–2.
- Elfers RS, Bayly WM, Brobst DF, Reed SM, Liggitt HD, Hawker CD, et al. Alterations in calcium, phosphorus and C-terminal parathyroid hormone levels in equine acute renal disease. Cornell Vet. 1986 Jul;76(3):317–29.
- Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg. 2006 Mar;191(3):418–23.
