Urine specific gravity (USG) and osmolality are measures of the solute concentration in urine and are used to assess tubular function, i.e. the ability of the renal tubules to dilute (loop of Henle) or concentrate (distal tubules) the glomerular filtrate.
Knowledge of urinary solute concentration is essential for proper interpretation of urea and creatinine, which are indicators of glomerular filtration rate. A wide USG range is possible in healthy euhydrated animals. However animals that are dehydrated, hypovolemic or have decreased effective blood circulating volume should be conserving water (and trying to reconstitute effective blood volume), therefore concentrating their urine. Thus, in the setting of azotemia or an increased urea nitrogen and/or creatinine concentrations, USG is used to determine whether concentrating ability is adequate and is very useful for distinguishing between causes of azotemia.
In order for the kidney to conserve water by concentrating urine, the kidney needs the following:
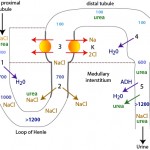
- Antidiuretic hormone (ADH): This is produced by the hypothalamus and stored in the posterior pituitary. ADH opens water pores (aquaporins) in the distal convoluted and collecting tubules allowing for water to be absorbed passively along a concentration gradient established by a hypertonic medulla. This concentrates the urine. ADH also promotes urea absorption, which contributes to medullary interstitial tonicity. Central diabetes insipidus results from lack of ADH, the consequence of which is usually hyposthenuric urine (urine that cannot be concentrated; USG < 1.008).
- Distal/collecting tubules must be responsive to ADH: Typically ADH works by opening up water channels, specifically aquaporin-2 (aquapore = water pore) in the collecting ducts (Boone and Deen 2008). In renal disease, damaged tubular cells are less responsive to ADH, which impairs renal concentrating ability (leading to “inadequately concentrated” or isosthenuric urine in animals with renal azotemia). Other extrarenal factors can also result in decreased responsiveness to ADH and these include: Hypercalcemia (various mechanisms proposed, but decreased expression of aquaporin-2 is involved [Khositseth et al 2017), corticosteroids (unknown mechanism), endotoxins (downregulate vasopressin receptors), and hypokalemia (downregulates aquaporin-2 [Marples et al 1996]). When tubules are not responsive to ADH (from primary tubular disease or extrarenal factors), it is called nephrogenic diabetes insipidus.
- A hypertonic medullary interstitium: Even with aquaporins in place in the collecting tubular cells, water will not be reabsorbed if the medulla is not hypertonic. A hypertonic medulla requires adequate amounts of sodium and urea (to create medullary hypertonicity), functioning tubules (proximal and loop of Henle) to deliver Na and urea to the renal medulla, and the countercurrent exchange mechanism maintained by medullary blood flow through the vasa recta. Defects in any of these can cause decreased urine concentrating ability. Therefore, the following can result in decreased medullary tonicity and decreased concentration ability:
- Decreased transport of Na and Cl from the ascending loop of Henle to the medullary interstitium (e.g. renal tubular disease, loop diuretics).
- Hyponatremia resulting in decreased filtered sodium and less available to be absorbed and transported to the medulla (e.g. electrolyte losses in diarrhea).
- Decreased production of urea resulting in decreased filtered urea available to be transported to the medulla in the descending limb of the loop of Henle and collecting tubules (e.g. liver insufficiency).
- Increased urine flow rate resulting in impaired reabsorption of Na, Cl and urea (e.g. osmotic or chemical diuresis such as due to diabetes mellitus or excess corticosteroids). Also called medullary solute washout.
- Increased medullary blood flow in vasa recta: This flushes out the solutes accumulating and creating hypertonicity in the medulla. Hypokalemia and hypercalcemia can both cause this effect. Also called medullary solute washout.
For more on how the kidney concentrates urine, refer to the renal physiology page.
The interpretation of several urine chemical parameters, such as protein and bilirubin, is also influenced by the specific gravity of the specimen. In addition, urinary constituents (erythrocytes, leukocytes and casts) can lyse in dilute urine (USG < 1.008), affecting interpretation of the urine sediment results.
Urine specific gravity
Urine specific gravity is a measurement of the density of urine compared to pure water. For routine clinical purposes, USG is determined using a refractometer (refractive index generally correlates well with USG). USG is influenced by the number of molecules in urine, as well as their molecular weight and size, therefore it only approximates solute concentration. It is also affected by temperature, with urine density decreasing (lower USG) with increasing temperatures. Urine specific gravity of commonly used optical and a digital refractometer show a strong correlation to urine osmolality (Spearman rank correlation coefficients around 0.94) (Rudinsky et al 2019). Cornell University uses a temperature-compensated Reichert refractometer or digital refractometers for USG measurements in animals. Urine color can provide a rough guide as to the expected USG, with increasing USG seen with increased intensity of yellow (e.g. colorless to very pale yellow urine usually has a USG <1.030 and dark urine usually has a USG >1.020) (Cridge et al 2018), however color is not a surrogate for USG measurement.
Factors affecting USG other than concentrating ability
- Protein and glucose: The presence of large amounts of protein and glucose will alter the USG and should be considered when interpreting USG results. The effect of these substances on USG does depend on the refractometer, some of which are more accurate than others (Tvedten et al 2015).
A concentration of 1 g/dL of the following substances in urine has been reported to increase the USG as follows:
|
Substance
|
Increase in USG
|
|
NaCl
|
0.006-0.007a
|
|
Urea
|
0.002-0.003a
|
|
Glucose
|
0.003-0.005a
-0.002 – 0.004 (dogs)b
00.008-0.007 (cats)b
|
|
Protein
|
0.003-0.005a
|
|
Albumin
|
0.002-0.003a
|
|
a Fundamentals in Veterinary Clinical Pathology (Stockham and Scott)
b USG dependent; Behrend et al 2019 (see below)
|
|
-
- Glucose: A study in which glucose was spiked into pooled canine and feline urine of different USGs showed the following:
- Canine urine: Addition of 300 mg/dL glucose increased the USG by 0.001-0.002 in samples with a USG < 1.025. Addition of 1200 mg/dL glucose increased the USG from 0.001-0.004 in samples with USG <1.025 (little effect was seen in higher USGs) and 2400 mg/dL glucose increased the USG >0.004 in samples with USG <1.020. With the latter highest glucose concentration, smaller increases were seen in samples with a USG 1.020 and 1.35 (-0.001 to 0.003) and the USG decreased in samples with a starting USG >1.040. There was a linear relationship between the difference in USG before and after spiking and the initial USG concentration (r2 ranging from 0.78 to 0.98 for glucose concentrations between 1200 and 2400 g/dL). Although these changes may not increase the USG into the “appropriate range” nor substantially interfere with distinguishing between prerenal and renal azotemia with the USG, it will interfere with determination of hyposthenuric and isosthenuric USGs.
- Feline urine: Addition of 50-300 mg/dL had random effects (increase and decrease of USG of 0.007) in individual cats with USG > 1.030. A decreasing linear relationship became evident when urine was spiked with 600 – 2400 mg/dL, but there was more individual cat variation (leading to r2 ranging from 0.26 to 0.48 for these glucose concentrations). At a concentration of 2400 mg/dL, the USG increased by 0.002 to 0.008 in pooled feline urine with USG < 1.030 (with a greater increase seen at the lower USG). In 4 pooled urine samples with an unspiked USG of 1.040, the USG decreased by 0.001 to 0.004. As stated above, the impact of glucose on allowing discrimination between prerenal and renal azotemia in cats is questionable. The authors of this study did not state the variability in refractometer readings with one urine sample (intra-assay variation) (Behrend et al 2019).
- Glucose: A study in which glucose was spiked into pooled canine and feline urine of different USGs showed the following:
- Radiographic contrast agents: In addition, the USG but not osmolality may be falsely increased (>1.10) with radiographic contrast agents, such as iohexol, which are eliminated in the urine typically within 24-48 hours after contrast imaging studies (Seshia and Dickinson 2020).
USG interpretation
First morning urine samples are frequently recommended when evaluating USG in dogs (it is believed that this would represent the “most naturally concentrated” urine sample. One study showed that the first morning urine sample of clinically healthy dogs ranged from as low as 1.010 to >1.060 in individual dogs and that the first morning urine varied by as high as 0.015 units (minimum to maximum) in different samples collected from the same dog over 2 weeks (within dog variability). Measurements of GFR or serum biochemical analytes of GFR was not done in these dogs (Rudinsky et al 2019).
Indicated below are guidelines for interpreting the USG in animals. Note that different cut-offs for “adequate” concentrating ability and isosthenuria are reported in the literature. Given below are the ones used here at Cornell University.
| Species | Possible range | Usual range | “Adequate” | “Inadequate” |
| Canine | 1.001-1.065 | 1.015-1.045 | >1.030 | < 1.030 |
| Feline | 1.001-1.085 | 1.035-1.060 | >1.040 | < 1.040 |
| Large Animals | 1.001-1.050 | 1.015-1.030 | >1.025 | < 1.025 |
- “Adequate” USG: The “adequate” USG or concentrating ability column is used specifically in azotemic animals. In this context, an “adequate” USG indicates the existence of sufficient functional nephrons to adequately concentrate the urine and to prevent development of azotemia, provided that renal blood flow is sufficient and that the ability of those nephrons to concentrate urine is not impaired by other factors, such as medullary solute washout (see above and renal physiology page), an adequate USG in an azotemic animal usually indicates a pre-renal azotemia.
- “Inadequate” USG: In azotemic animals with primary nephropathies characterized by progressive loss of of functional nephrons, the ability to concentrate urine is compromised when about two-thirds of the nephron mass is lost. However, clearance of nitrogenous waste products sufficient to prevent azotemia, persists until roughly three-quarters of functional nephrons are lost. Therefore, if azotemia is due to loss of nephron mass (> three-quarters loss, i.e., renal failure), ability to concentrate urine will have already been lost (i.e. the USG will be less than “adequate” for that species). An exception to this occurs in cats, in which glomerular disease (and azotemia) can precede loss of concentrating ability. Thus, an “inadequate” USG in an azotemic animal is compatible with renal disease and a renal azotemia unless there are other factors that impair the ability of the kidney to concentrate, including decreased hypertonicity of the medullary interstitium and inhibition of ADH (see above and renal physiology page).
- Isosthenuria: In a primary renal azotemia, the kidney cannot concentrate or dilute urine, so there is often a fixed (constant) isosthenuric USG, i.e. USG of 1.008-1.012. However, in renal disease, the total loss of renal tubule function occurs gradually, therefore USGs between isosthenuric and “adequate” ranges in animals with dehydration and/or azotemia, are highly suggestive of primary renal failure.
- Hyposthenuria: Hyposthenuria indicates that the kidney can dilute the urine but is unable to concentrate, i.e. proximal renal tubule and loop of Henle function is retained but the connecting tubules are unresponsive to ADH, either from a primary ADH deficiency (central diabetes insipidus) or lack of responsiveness of renal tubules to ADH due to renal tubular disease or inhibitors of ADH (nephrogenic diabetes insipidus).
Urine osmolality
Urine osmolality is directly related to the number of particles in solution and is unaffected by molecular weight and size. For this reason, osmolality is superior to specific gravity, which is affected by particle weight and size. Osmolality can be measured by freezing point depression (the technique used at the Clinical Pathology Laboratory of the Animal Health Diagnostic Center at Cornell University) and changes in vapor pressure. Urine osmolality can also be approximated from the USG. This is calculated by multiplying the last two digits of the USG by 36. Although urine specific gravity correlates well to urine osmolality, the osmolality cannot be accurately predicted from the USG, i.e. the same USG can yield very different urine osmolalities (Rudinsky et al 2019).
Isosthenuric urine has an osmolality similar to plasma, approximately 300 to 320 mOsm/kg. Urine osmolality is useful for evaluating urine concentrating ability, for example in water deprivation tests, and is more accurate than measurement of urine specific gravity in this regard.
Related links
- Renal physiology page: Provides more detailed information on how the kidney maintains a concentrated medulla, how ADH works to cause anti-diuresis, and factors that interfere with this process.
