Interpretation
Neoplastic effusion
Explanation
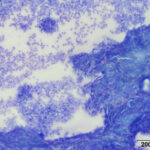
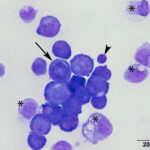
A direct smear prepared from the submitted fluid (not shown) was of low cellularity containing few macrophages, non-degenerate neutrophils, and individualized mesothelial cells on a clear background. In contrast, the squash smear prepared from visible “clots” in the fluid was highly cellular. This smear contained abundant macrophages (Figure 5B), as well as multiple large, cohesive rafts of cells with small amounts of admixed pale pink extracellular matrix and thin vascular profiles (Figure 1). The automated cell counts were not reported as they were considered falsely low and inaccurate, as these large clusters would have been excluded from the nucleated cell count.
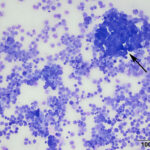
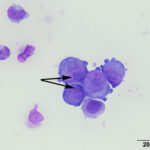
At low power, there were clusters of cells with a distinctive morphologic appearance (Figure 2B). The cells in the clusters were round with a high nuclear to cytoplasmic ratio and deep blue cytoplasm, and occasionally had blebbed cell borders. These cells displayed cytologic criteria of malignancy in the form multiple prominent nucleoli, moderate anisocytosis and anisokaryosis, and mitotic figures (Figures 4B and 5B). The cytologic interpretation was a neoplastic effusion with mesothelioma and carcinoma as the two differential diagnoses. Cytology alone cannot reliably differentiate these two neoplasms, however a mass lesion was not identified on ultrasonographic examination of the abdomen (the latter would favor a carcinoma).
Follow-up
Humane euthanasia was elected, in lieu of abdominal exploratory surgery, due to the poor prognosis. A necropsy was performed. On gross examination, there was severe peritoneal effusion (10 L), as well as a moderate pleural (750 mL) and pericardial (20 mL) serosanguinous effusion. There was severe hardening of the adipose tissue within the abdomen and thorax, which on histopathologic examination was determined to be a result of fat necrosis and saponification, a cause for which was unclear. No masses were evident on gross examination.
On histopathologic examination of the jejunum, the serosa was found to be severely expanded by severely pleomorphic mesothelial cells arranged in sheets and vague herringbone streams. Marked anisocytosis and anisokaryosis, binucleation, multinucleation, and 12 mitotic figures (most of which were bizarre) per ten 400x fields were present. A diagnosis of mesothelioma was made at this site. A proliferation of dysplastic mesothelial cells was also noted overlying the urinary bladder, abomasum, and abdominal fat.
Discussion
Mesothelioma is a malignant tumor that arises from mesothelial cells, which line the body’s serous cavities (pericardial, pleural, and peritoneal) and the internal organs within these cavities. Mesothelioma is rare in the goat with only a handful of case reports.1–3 It was also not reported in a 25 year retrospective of one hundred and two tumors in 100 goats at a single institution.4
Mesothelial cells form a monolayer, known as the mesothelium, the primary function of which is to protect the organs in the body cavities and allow them to freely move across a frictionless surface. These cells have other functions, including transport of fluids and cells, antigen presentation, inflammation and tissue repair, coagulation, fibrinolysis, and tumor cell adhesion. One of the ways in which fluids and cells are transported across serosal surface is through stomata, or openings between mesothelial cells, leading to underlying lymphatics, which rapidly remove these substances.5 With this function in mind, it is understandable why effusions form as a consequence of a proliferative mesothelium, as occurs with neoplastic transformation of these cells.
Mesothelioma results in fluid accumulation in the body cavities in which it is present. It can occur in one, two, or all three cavities.1–3,6 Abdominal enlargement, as well as non-specific signs such as lethargy and inappetence or anorexia, are common presenting complaints in cases of peritoneal mesothelioma.2,3,7 Dyspnea and tachypnea are seen with pleural mesothelioma1 and collapse can be seen with pericardial involvement6 due to cardiac tamponade resulting in decreased cardiac output. There is often a significant volume of ascites with peritoneal mesothelioma. The case presented here had 10 L of peritoneal fluid, surpassing both of the reported caprine peritoneal cases in the literature (4 L3 and 7 L2). The fluid is often slightly cloudy, and can appear yellow or red-tinged.
Peritoneal fluid analysis with cytologic examination is a minimally invasive diagnostic test that can help exclude sterile or septic inflammatory exudates. Cytologic distinction between reactive and neoplastic mesothelial cells can be challenging, particularly in the pericardial cavity, where mesothelial cells typically display a high degree of atypia. However, a highly cellular sample forming large clusters, as present in this case, with minimal inflammation certainly raises the index of suspicion for neoplasia. This finding, in conjunction with cytologic criteria of malignancy and a supportive clinical history, can often lead to a diagnosis of a neoplastic effusion. However, the distinction between mesothelioma and carcinoma cannot reliably be made on cytologic examination alone. The clinical history (e.g. presence of a mass lesion, single or multiple cavity effusion) can result in favoring of one differential diagnosis over the other. While the presence of a mass lesion favors a carcinoma, mesotheliomas can reportedly present as a single distinct large mass.7 More often, on gross necropsy, mesotheliomas appear as multiple, small (1mm – 3cm), white, grey, tan or red, soft to firm, nodules on the cavity surface and serosal surface of organs.1–3,6 Carcinomatosis can appear similar, however.
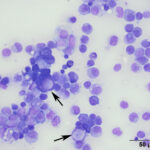
Even on histopathologic examination, the distinction between reactive and neoplastic mesothelial cells can be challenging. Given that reactive mesothelial cells can display individual cell atypia, a definitive diagnosis relies on demonstration of invasion into the underlying tissue.7,8 There are several histologic patterns of mesothelioma including epithelioid, sarcomatoid, and biphasic, in which mesothelial cells can take on an epithelial, mesenchymal, or mixed appearance.8 The epithelioid type is the most common.8 The presumptive neoplastic cells in the cytologic sample presented here were compatible with what has been reported with the epithelioid type, including some cells with a signet-ring appearance (Figure 3B).6 Signet-ring cells are often associated with carcinomas, however this case demonstrates they can be seen with mesotheliomas, as well. In human medicine, each category of mesothelioma is further divided into multiple subtypes/patterns, reflecting the variety of histologic patterns they can take on, including papillary, trabecular, solid, desmoplastic, etc.8 This raises the awareness that while carcinomas are often thought of as having the potential for a scirrhous response, mesotheliomas can also manifest as few neoplastic cells with abundant non-neoplastic fibrous tissue, which may include foci of cartilage or bone.7 Therefore with the variety of histologic patterns possible, it is often necessary to use immunohistochemistry to reach a definitive diagnosis. Normal and neoplastic mesothelial cells co-express cytokeratin and vimentin, therefore this dual positivity can be used to support a diagnosis of mesothelioma and help rule out a carcinoma or sarcoma, respectively.2,3,5,6,8 Note, that other tumors (e.g. ovarian and renal carcinoma) can rarely co-express these markers, therefore a thorough evaluation for a primary tumor is necessary.7 While calretinin is also reportedly positive in humans mesotheliomas,7,8 this marker has been shown to be inconsistent and unreliable in veterinary medicine.9 Electron microscopy can also be performed to demonstrate microvilli, characteristic of mesothelial cells,9 however these microvilli are not present in sarcomatous mesotheliomas.7
In humans, mesotheliomas are mainly attributed to exposure to asbestos, which is thought to result in a variety of carcinogenic effects (e.g. chromosomal aberrations, deletions in tumor suppressor genes). Simian virus 40 has also been recognized as a causative agent for this tumor in rodents, with mesotheliomas developing following injection of the virus into visceral cavities. While the duration from exposure to diagnosis of mesothelioma in humans is long (up to 40 years), patients typically die within 18 months of diagnosis.5 Prognosis and survival times are difficult to gauge in veterinary medicine, as most animals are euthanized at the time of diagnosis,1,3,7 as in this case. However, in one of the published case reports of a peritoneal mesothelioma in a goat, the animal died en route to the hospital, after only a one week history of clinical signs.2 The tumor is typically considered malignant, given its presence in a fluid medium, which facilitates the spread of neoplastic cells within the body cavity to the surfaces of distant organs.
References
1. McCullagh KG, Mews AR, Pinsent PJ. Diffuse pleural mesothelioma in a goat. Vet Pathol. 1979;16(1):119–21.
2. Ruby RE, Daverio H, Barrell EA, Southard TL. Pathology in Practice. J Am Vet Med Assoc. 2016;248(1):63–5.
3. Krametter R, Bagó Z, Floeck M, Baumgartner W. Abdominal mesothelioma in a goat. N Z Vet J. 2004;52(5):293–6.
4. Löhr C V. One hundred two tumors in 100 goats (1987-2011). Vet Pathol. 2013;50(4):668–75.
5. Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol. 2004;36(1):9–16.
6. Reggeti F, Brisson B, Ruotsalo K, Southorn E, Bienzle D. Invasive epithelial mesothelioma in a dog. Vet Pathol. 2005;42(1):77–81.
7. Munday JS, Lohr C V., Kiupel M. Tumors of the Alimentary Tract. In: Meuten DJ, editor. Tumors in Domestic Animals. Fifth edit. Ames, Iowa: Wiley-Blackwell; 2017. p. 592–5.
8. Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2012 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2012;137(5):647–67.
9. Schappa JT, Foutz CA, Olson EJ, Armien AG, Ward C, Sharkey LC. What is your diagnosis? Bicavitary effusion in a horse. Vet Clin Pathol. 2017;46(1):189–90.
Authored by: A. Newman