Interpretation
Acute leukemia, suspect acute myeloid leukemia, with concurrent myelodysplasia
Explanation
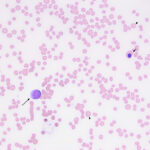
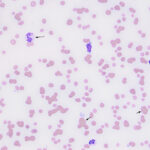
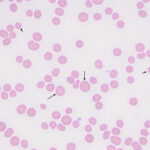
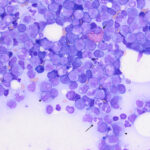
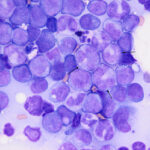
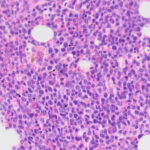
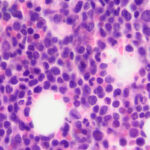
Examination of a blood smear revealed dysplastic neutrophils (giant forms [Figure 2], hypersegmentation), and rare circulating blasts (Figure 1). The blasts were intermediate to large cells with round nuclei containing fine chromatin and indistinct nucleoli. They had a small amount of light to medium blue cytoplasm (Figure 1). There was moderate anisocytosis in red blood cells due to the presence of moderate macrocytes. Rouleaux formation and mild agglutinates were present (Figure 3). A few nucleated red blood cells were seen on scanning, some of which were dysplastic (Figure 1). There were smaller spherocyte-like red blood cells in the smear (Figure 3), however examination of a wet preparation of a saline dilution of the blood smear did not support that they were true spherocytes (spherocytes are usually spherical cells with small indentations in such preparations). Platelet clumps were noted in the blood smear but the count was still considered decreased (Question 1). The pancytopenia with circulating blasts supported an underlying acute leukemia, of lymphoid or myeloid origin. The dysplasia in the neutrophils and red blood cells favored an acute myeloid leukemia (AML) with concurrent myelodysplasia (Question 3), however further phenotyping was required to confirm this suspicion. Although we look for macrocytes to support a regenerative response to an anemia, in this case, due to the dysplasia, the macrocytosis was attributed to abnormal red blood cell production versus a regenerative response (Question 2). There were sufficient macrocytes in the blood to result in a macrocytosis in this horse. The high red blood cell distribution width was also attributed to the macrocytosis. The mild hyperglobulinemia with a normal fibrinogen concentration supported an increase in immunoglobulins as the cause for rouleaux formation.
|
|
|
|
Additional tests
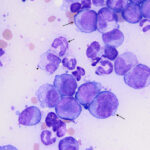
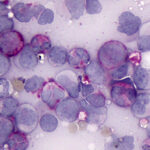
Agglutinates were still present in wet preparation of the saline dilution of blood. Given that a direct Coomb’s test on the EDTA-anticoagulated blood was negative, the agglutinates could reflect the presence of a cold agglutinin (the blood was not warmed to check for this possibility). A Coggins test (ELISA for Equine Infectious Anemia Virus) and PCR test for Anaplasma phagocytophilum were negative. To confirm the suspicion of an acute leukemia, a bone marrow aspirate was obtained from the sternum and smears of the bone marrow were stained with modified Wright’s stain. Bone marrow cellularity was markedly increased but only low numbers of mature megakaryocytes were seen. The majority of cells (60% based on a differential cell count) contributing to the hypercellularity were a homogenous population of large, round, mononuclear cells (“blasts”) with a high nuclear to cytoplasmic ratio, grainy blue cytoplasm, round-to-oval-to0irregular nuclei with fine chromatin and 1-3 nucleoli (Figures 4-7). The remaining cells consisted of 28% differentiating and mature monocytes, 5% differentiating neutrophil precursors, 2% mature segmented neutrophils, 2% differentiating eosinophils, and 3% erythroid precursors. Monocytes (Figure 5), neutrophils (Figure 6) and erythroid precursors were dysplastic. A few polychromatophilic red blood cells had light blue cytoplasmic inclusions (presumptive siderocytes; not conclusively identified in a Prussian blue stain). Low numbers of plasma cells and cytophagic macrophages were seen on scanning. Marrow iron was judged markedly decreased on a Prussian blue stain. The cytologic diagnosis was an acute leukemia, presumptive myeloid with evidence of monocytic differentiation, and concurrent myelodysplasia. The decreased iron stores was attributed to increased use by the tumor cells. The peripheral cytopenias were attributed to myelophthisis and ineffective hematopoiesis due to concurrent dysplasia. The high iron concentration and % saturation of transferrin in serum can be attributed to increased iron turnover from ineffective erythropoiesis.
Cytochemical staining was performed on unstained bone marrow smears. The blasts were positive for chloroacetate esterase (CAE, 14%, Figure 8) and Sudan Black B (SBB, 7%) but were unexpectedly negative for alpha-naphthyl butyrate esterase (ANBE, a monocyte marker; monocytes in a control horse blood stained as expected, i.e. diffuse staining in monocytes). Only 2% of the blasts were positive for myeloperoxidase (a 3% cut-off is used for cytochemical staining) and they were negative for alkaline phosphatase, which stains mature neutrophils and eosinophils. Neutrophils and eosinophils in the marrow were variably positive for CAE. The cytochemical results supported an acute myeloid leukemia (AML), but could not confirm a monocytic lineage for the tumor cells, although CAE (Figure 8) and SBB can be expressed by monocytes, despite being considered more typical of neutrophils. Given the apparent morphologic monocytic differentiation of the blasts, an explanation for the lack of ANBE staining was not apparent, but it could be related to the neoplastic transformation of the cells.
Outcome
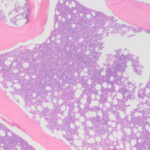
Due to the poor prognosis, the horse was humanely euthanized. A post-mortem examination revealed nodules in the liver, a tan (hypercellular) bone marrow, and mild to moderate lymphadenopathy. Histologic examination showed marked hyperplasia of lymphocytes in the lymph nodes, pronounced lymphoid follicle formation in the liver, mimicking lymphoma (pseudolymphoma), and a hypercellular marrow with increased numbers of large round cells, compatible with an acute leukemia (Figures 9-11). Tumor infiltrates were identified in the lung (interstitial infiltrates cuffing vessels) and kidney (multifocal interstitial infiltrates in a perivascular location). Over 90% of the cells in the marrow were positive for Iba-1 (a histiocytic marker, which can also be positive in AML1,2) and 80% were positive for myeloperoxidase. They were negative for T (CD3) and B (CD79a) cell markers. The lymphoid markers confirmed the mixed lineage of the lymphoid expansions in the liver and lymph nodes. Tumor cell infiltrates were also identified, using the latter two stains, in the lymphoid follicles in the liver and were scattered in the medulla and cortex of the lymph nodes, portal adventitia of the liver, and were found as clusters in the red and white pulp of the spleen. The final diagnosis was AML with a reactive lymphoid hyperplasia.
Discussion
Acute myeloid leukemia (AML) is rare in horses, this case being one of a handful that have been described in recent years.1-3 The lack of published data leads to gaps in our knowledge about this hematopoietic neoplasm in horses. As the name implies, AML is a neoplasm that arises from the hematopoietic stem cell but differentiates down myeloid lineages – megakaryocytic, erythroid, monocytic, or granulocytic (usually neutrophilic) or a combination of these (typically myelomonocytic).4,5 The cellular differentiation can range from minimal to full with cells mostly being identified as large and immature cells (blasts) when minimally differentiated. These neoplastic cells typically arise in and proliferate in the bone marrow, where they disrupt normal hematopoiesis, resulting in peripheral cytopenias.1,2,4,5 AML in horses is usually classified into types based on their expression of differentiation markers.1,2,6 In humans, we now know that genetic variants of AML differ in biological behavior and prognosis, with specific gene mutations being definitive for AML,7 however, we currently have no knowledge of the genetic mutations underlying AML in horses. The most common subtype of AML in horses is acute myelomonocytic leukemia, based on morphologic features and phenotyping (immunophenotyping with flow cytometry, cytochemical staining, immunohistochemical staining).2,5,6 In this case, a monocytic lineage was suspected (acute monocytic leukemia), based on 26% differentiating monocytes in bone marrow aspirate smears. This classification was supported by the Iba-1 immunostaining results on the bone marrow sections from post-mortem, despite the lack of cytochemical staining for ANBE.
Based on published reports, there is no obvious breed, sex, or age predisposition for AML in horses. AML has been reported in horses as young as the case herein, although most of the cases are in older animals.1,2 Presenting clinical signs are non-specific and typically of acute onset (anywhere from a few days to two months) and include fever, lethargy, weakness, and inappetence.1,2 On physical examination, horses are often pyrexic, tachycardic, and tachypneic, and may have peripheral lymphadenopathy or edema.1,2 While the history and physical examination findings do not elucidate much regarding the definitive diagnosis, there are several key findings on bloodwork that can point towards an acute leukemia (or at least a disorder within the bone marrow). Horses with AML usually have moderate-to-severe bi- or pancytopenia.1,2 Blood smears can reveal low numbers of circulating blasts, occasional nucleated red blood cells, and dysplastic features in one or more lineage.1,2 While these hematological findings should be concerning for an acute leukemia, a bone marrow aspirate is required to make a diagnosis. Acute leukemia is definitively diagnosed by identifying >20% blasts in a bone marrow aspirate.1,2,5 Accordingly, in horses with AML, the bone marrow aspirates are highly cellular and the percentage of blasts have been reported to range from 32% to 98%, with the remaining hematopoietic lineages exhibiting hypoplasia.1,2,6 Dysplasia can be seen in some cases1,3 and differentiation without an effaced marrow is more typical of this leukemia in horses2 than dogs, with the latter species usually having >80% blasts by the time an aspirate is performed.8 Biochemical test abnormalities are not typically helpful and are not specific, most commonly showing evidence of inflammation, with an increase in acute phase protein concentrations, such as serum amyloid A, and globulins,1,2 including fibrinogen and immunoglobulins (typically polyclonal)1. Lymphoid reactivity can be quite pronounced and mimic lymphoma in some cases, which seems to be a unique finding in horses versus other animal species (Bienzle D, personal communication). Immunohistochemical staining with lymphoid markers, however, would reveal a mixed lymphocyte population, as seen in this case, and serum total protein electrophoresis, if performed, would reveal a polyclonal gammopathy in horses with hyperglobulinemia.1 Histologic examination of post-mortem tissues of horses with AML have revealed, in addition to the expected bone marrow involvement, extramedullary infiltrates of neoplastic cells within several organs, including the lymph nodes, kidneys, spleen, liver, lungs, and brain.1,2,9 The case herein had tumor infiltrates in the lungs, kidney, spleen and liver, but they were only definitively identified with immunohistochemical staining for myeloid antigens.
After reaching a preliminary diagnosis of acute leukemia through blood and bone marrow analysis, the next step is to determine the lineage (myeloid vs lymphoid) via flow cytometric immunophenotyping and cytochemical staining. Distinguishing AML from lymphoid neoplasms can be challenging, as cross-lineage expression of lymphoid antigens and aberrant expression of lymphoid markers on the myeloid cells can occur in AML and myeloid markers can be expressed in lymphoid neoplasms.1,10,11 Due to the lack of defining genetic mutations, in equine medicine we use an approach of casting a wide net, applying as many immunologic markers and cytochemical stains as possible, and interpreting the resulting data in conjunction with the morphologic features of the cells to arrive at a diagnosis.1,2,3,6 In this case, we only performed cytochemical staining on the tumor cells, which yielded unexpected results (for ANBE) given morphologic features of the cells, and immunohistochemical staining for myeloperoxidase, Iba-1, CD3 and CD79a, which is a limited set of antigens (more myeloid markers being available with flow cytometry1,2.)
Acute myeloid leukemia carries a grave prognosis in horses , with most animals being euthanized or dying within days of their diagnosis.1,2 Therapies such as glucocorticoids and chemotherapeutic agents (vincristine, cytarabine, etc.) have been attempted, but thus far no reported treatment has been successful.1,2,3,6,9
Authors: Colette Angel DVM and Tracy Stokol
References
- Barrell EA, Asakawa MG, Felippe MJB, Divers TJ, Stokol T. Acute leukemia in six horses (1990-2012). J Vet Diagn Invest 2017;29(4):529-535.
- Cooper CJ, Keller SM, Arroyo LG, Hewson J, Kenney D, Bienzle D. Acute leukemia in horses. Vet Pathol 2018;55(1):159-172.
- Miglio A, Pepe M, Felippe MJB, Antognoni MT. Subleukaemic acute myeloid leukaemia with myelodysplasia in a horse. Equine Vet Educ 2019;31:e39-46.
- Raskin RE (editorial). Diagnostic tools and dilemmas with equine leukemias. Vet Pathol 2018;55(1):11-13.
- Satué K, Gardon JC, Muñoz A. A review of current knowledge of myeloproliferative disorders in the horse. Acta Vet Scand 2021;63:8.
- McClure JT, Young KM, Fiste M, Sharkey LC, Lunn DP. Immunophenotypic classification of leukemia in 3 horses. J Vet Intern Med 2001;15(2):144-52.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127(20):2391-2405.
- Davis LL, Hume KR, Stokol T. A retrospective review of acute myeloid leukaemia in 35 dogs diagnosed by a combination of morphologic findings, flow cytometric immunophenotyping and cytochemical staining results (2007-2015). Vet Comp Oncol 2018;16(2):268-275.
- Ringger NC, Edens L, Bain P, et al. Acute myelogenous leukaemia in a mare. Aust Vet J 1997;75(5):329-331.
- Stokol T, Nickerson GA, Shuman M, Belcher N. Dogs with acute myeloid leukemia have clonal rearrangements in T and B cell receptors. Front Vet Sci 2017;4:76.
- Suggs JL, Cruse JM, Lewis RE. Aberrant myeloid marker expression in precursor B-cell and T-cell leukemias. Exp Mol Path 2007;83:471-473.