Interpretation
Mixed, mostly neutrophilic, septic inflammation
Explanation
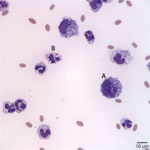
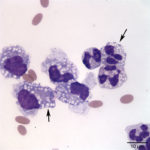
The direct and sediment smears were of moderate cellularity, consisting of mixed inflammatory cells, on a background of a small amount of blood. A 100-cell differential yielded 80% non-degenerate neutrophils (Question 2, cell labeled as “B”), and 20% macrophages (Question 2, cell labeled as “A”). Both neutrophils and macrophages contained moderate numbers of discrete, margined vacuoles, compatible with lipid (Figure 2 and 3). The marginally increased total protein (>2.5 g/dL) and nucleated cell count of 6.5 thousand/µL (reported upper limit, 3 thousand/µL1) and the increased neutrophils (>65%1) indicated inflammation. Rare intracellular and extracellular Gram positive and negative rods (Figure 4 and 4a, arrow) were seen.
The inflammatory cells contained clear, discrete margined cytoplasmic vacuoles (Figure 2 and 3), suggesting lipid ingestion. Effusions containing inflammatory cells with phagocytized lipid are typically lymphocyte-rich, chylous effusions.2–4 In this case, the effusion was predominantly neutrophilic and no lymphocytes were observed, thus cytologic findings were not compatible with a chylous peritonitis. However, similar lipid vacuolization has been associated with steatitis and necrosis of fat secondary to pancreatitis or bile peritonitis.6,4 To rule out these differential diagnoses, activities of pancreatic enzymes, amylase and lipase, and total bilirubin concentrations were determined on peritoneal fluid and serum (Question 3). Peritoneal fluid amylase (826 U/L) and lipase (13 U/L) activities were lower than in serum (amylase, 993 U/L; lipase, 70 U/L). These results made pancreatitis as the cause of the peritonitis (and lipid vacuoles in peritoneal fluid cells) less likely.6 Total bilirubin concentration was, however, 6 times greater in the peritoneal fluid (0.6 mg/dL) than in serum (0.1 mg/dL) supporting a bile peritonitis.2,4
Biochemistry results showed a hypochloremic metabolic alkalosis (Question 1). The disproportionate high sodium compared to chloride, and low corrected chloride (calculated value, 101 mEq/L) indicated loss or sequestration of chloride (in the form of HCl–) in excess of sodium. In this case, the hypochloremic metabolic alkalosis was interpreted as primary due to HCl- sequestration in the upper gastrointestinal tract related to the outflow obstruction caused by the bezoars (C3 and duodenum).
Discussion
Bile peritonitis in New World camelids is uncommon and to the author’s knowledge there are no previous reports of bile peritonitis on alpacas.5 In small animals, bile peritonitis occurs due to rupture of the gall bladder or common bile duct. Rupture may be due to biliary tract obstruction (e.g. cholestasis, cholelitiasis), inflammation, trauma (e.g. gastric dilatation, volvulus, gunshot wound), mucocele, or iatrogenic (surgery or fine needle aspiration).3,2
Bile is an irritating substance, and in the peritoneal cavity causes a chemical peritonitis that is typically exudative, as seen in this case.3 The fluid is typically green or yellow to orange in color. Cytologically, bile in effusions can be seen as an amorphous to granular blue- to yellow-green material within inflammatory cells or extracellularly. A variant of “normal” bile, so called “white bile”, consists of an amorphous, blue-gray mucinous material (see case of the month, April 2016), and is most commonly seen in dogs with rupture of the common bile duct or gall bladder mucoceles.6 The described cytologic findings in this case should raise concern for bile peritonitis. A helpful tool in the diagnosis of bile peritonitis is the determination of peritoneal fluid and serum total bilirubin. In small animal cases, peritoneal fluid bilirubin at least 2 times greater than serum bilirubin is diagnostic for bile peritonitis.3 Bile pigment was not observed on examined smears, but given the gross appearance of the fluid, the lipid vacuoles within the inflammatory cells, and the evidence of liver injury and likely biliary hyperplasia from the biochemistry results, we considered bile peritonitis a valid differential diagnosis. The diagnosis was supported by the 6 times greater total bilirubin in the peritoneal fluid compared to serum and the fact that one of the obstructing bezoars was near the bile duct.
Alpacas lack a gall bladder, thus the bile peritonitis in this patient likely resulted from a small tear (not grossly visible) of the bile duct as a consequence of the bezoar. It is possible that the duodenal bezoar led to a small perforation of the intestine with the subsequent release of intestinal constituents (including gastrointestinal bacteria and bile) into the peritoneal cavity. Alternatively, the marked distension of the third gastric compartment could have caused renting of the bile duct due to compression. Release of bile at the sites of obstruction during the surgical procedure cannot be ruled out as a cause of the increased bilirubin concentration in the fluid, but such an acute release of bile would not be expected to result in a peritonitis. It is also possible (and perhaps more likely, since food contents were not seen in the fluid) that the bacteria originated from bile.
The serum biochemical results indicated hepatobiliary disease, which is likely a consequence of ascending cholangiohepatitis (secondary from intestinal stasis secondary to the duodenal bezoar). The marked increased AST (normal CK), SDH, and GLDH activities indicate severe hepatocellular injury, whereas the marked increase in GGT activity likely reflects biliary hyperplasia, considering the total bilirubin was normal. Concurrent cholestasis cannot be ruled out (urine was not collected to evaluate for bilirubinuria, which may precede a bilirubinemia). The hepatocellular injury in this case could have also been exacerbated by hypoxic injury from hypoperfusion secondary to dehydration (supported by the hypernatremia). Dehydration was likely due to decreased water intake (given the animal was recumbent and anorectic) and fluid sequestration within the gastrointestinal tract. As stated above, the hypochloremic metabolic alkalosis in this alpaca was interpreted as a primary process associated with the proximal intestinal obstruction.
This alpaca presented with clinical signs suggestive of intestinal obstruction. Imaging and abdominal surgery confirmed this diagnosis and indicated the cause – bezoars. Obstructing bezoars commonly lodge at sites where the gastrointestinal tract narrows. The duodenal ampulla is the narrowest portion of the gastric tract and a common site of obstruction in New World camelids.7 Bezoars consist of either ingested plant material (phytobezoars), hair (trichobezoars), or a combination of both (trichophytoezoars).7 Obstruction by phytobezoars is common in camelids of all ages and <1 year old alpacas appear particularly predisposed to intestinal obstruction by tricho- and phytobezoars.8 Bezoar formation is not completely understood, but aberrant grooming behavior, pica, ectoparasites, dietary deficiencies, confinement, and weaning have been proposed as predisposing factors.7
The other differential diagnosis in this case was pancreatitis. There are few reports of pancreatitis due to acute pancreatic necrosis in New World camelids and they have conflicting results regarding its antemortem diagnosis.9,10,11 One retrospective study concluded that the only laboratory test that helps in the antemortem diagnosis of pancreatitis was comparison of pancreatic enzymes in peritoneal fluid and serum. This study showed that in 5 of 7 animals with pancreatic necrosis, peritoneal fluid amylase activity was higher than that in serum, and lipase activity was higher in peritoneal fluid than serum in all 7 animals. A report of pancreatic necrosis in a llama however, described that pancreatic enzymes were higher in serum than in peritoneal fluid.10 Based on these reports, the lower activity of amylase and lipase activity in the fluid compared to serum would not definitively rule out pancreatitis in this alpaca however, the higher total bilirubin in the fluid compared to serum was more compatible with bile peritonitis.3
Case follow up
The patient recovered from surgery without issues, and continued to improve during its hospital stay. The fluid was not submitted for bacterial culture, so the observed organisms could not be speciated. The alpaca was treated for its metabolic abnormalities, was given gastroprotectants, antibiotics, and analgesics and was discharged 6 days after initial admission to the care of its owners. Unfortunately, the patient passed away 10 days after discharge. A necropsy was performed, and the caused of death was determined to be severe peritonitis with secondary sepsis due to small intestinal dehiscence.
References
- Cebra CK, Tornquist SJ, Reed SK. Collection and analysis of peritoneal fluid from healthy llamas and alpacas. J Am Vet Med Assoc. 2008;232(9):1357–61.
- Thompson CA, Rebar AH. Body Cavity Fluids. In: Canine and Feline Cytology [Internet]. Third Edit. Elsevier Inc.; 2016. p. 191–219.
- Valenciano C A, Arndt P T, Rizzi E T. Effusion: Abdominal, Thoracic, and Pericardial. In: Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat. Fourth. St Louis, MO: Elsevier; 2013. p. 244–65.
- Meadows RL, MacWilliams PS. Chylous Effusions Revisited. Vet Clin Pathol. 1994;23(2):54–62.
- Cebra CK, Cebra ML, Garry FB, Larsen RS, Baxter GM. Acute gastrointestinal disease in 27 New World camelids: clinical and surgical findings. Vet Surg VS [Internet]. 1998;27(2):112–21.
- Owens SD, Gossett R, McElhaney MR, Christopher MM, Shelly SM. Three cases of canine bile peritonitis with mucinous material in abdominal fluid as the prominent cytologic finding. Vet Clin Pathol. 2003;32(3):114–20.
- Sullivan EK, Callan RJ, Holt TN, Van Metre DC. Trichophytobezoar duodenal obstruction in New World camelids. Vet Surg. 2005;34(5):524–9.
- Cebra C. Disorders of the Digestive System. In: Llama and Alpaca Care, Medicine, Surgery, Reproduction, Nutrition, and Herd Health. St Louis, MO: Elsevier; 2014. p. 477–536.
- Pearson EG, Snyder SP. Pancreatic necrosis in New World camelids: 11 cases (1990-1998). J Am Vet Med Assoc. 2000;217(2):241–4.
- Hamir A, Habecker P, Tilman C. Pancreatic necrosis in a llama. Vet Rec. 1998;142:644–5.
- Dickinson C, Pearson E, Lassen E. Characteristics of normal peritoneal fluid in new world camelids (abstr). In: 15th Annu Meet Vet Med Forum. 1997. p. 684.
Authored by: Drs. D. Hernandez (Clinical Pathology resident) and T Stokol. The authors would like to thank Dr. Annie Madison for providing follow up information for this case.