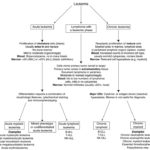
At Cornell University, we generally distinguish three different main types of leukemia: Acute leukemia, chronic leukemia and lymphoma with a leukemic phase. We use a combination of clinical signs, hematologic findings and immunophenotyping/cytochemical results for distinguishing between the types of leukemia (see algorithm). We have also provided this information in an introductory instructional video.
Acute leukemia
These leukemias generally arise primarily in the bone marrow and are due to genetic mutations that prevent cell maturation and promote proliferation. Thus, the neoplastic cells proliferate in and replace the bone marrow, so that normal hematopoietic cells are markedly decreased (called myelophthisis). The leukemic cells can also infiltrate extramedullary sites secondarily (spleen, liver, lymph nodes). The term “acute” applies to the stage of maturation, i.e. immature or precursor cell. In reality, acute leukemias (myeloid or lymphoid) are also acute in terms of chronology (rapid onset) and progression. They are frequently rapidly fatal, responding poorly to therapy (particularly myeloid variants). In general, the tumor cells have fine chromatin (euchromatin) and indistinct nucleoli, i.e. they are called “blasts”. They may or may not show features of differentiation so morphologic features should not be relied upon to differentiate acute myeloid leukemia (AML) from acute lymphoblastic/lymphoid leukemia (ALL) or acute leukemia from immature/precursor/lymphoblastic forms of lymphoma.
Animals with acute leukemia usually present with severe cytopenias (because the marrow has been replaced) and many circulating blasts, with mild splenomegaly, hepatomegaly and lymphadenopathy (there are exceptions to every rule). In some cases, very low numbers or no circulating blasts may be observed in peripheral blood smears. These latter patients are usually bi- or pancytopenic and bone marrow aspiration is required to identify the underlying cause for the cytopenias. This is typically the case in horses, where most animals with acute leukemia present with pancytopenia and low numbers to no circulating blasts.
The diagnosis of acute leukemia requires the identification of more than 20-25% blasts in the bone marrow, i.e. a bone marrow aspirate is essential to confirm the diagnosis. A cut-off of 20% blasts is used for the diagnosis of AML with a cut-off of 25% used to distinguish ALL from lymphoma (Swerdlow et al 2008). However, by the time we see most veterinary patients, blasts dominate in marrow comprising >70-80% of marrow cells.
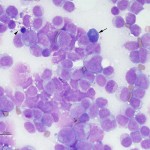
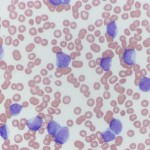
Acute leukemias can be of myeloid or lymphoid lineage, although there are undifferentiated or mixed phenotype leukemias (demonstrating markers of lymphoid and myeloid lineage). In our experience (Stokol et al 2015) and some published studies (Vernau and Moore, 1999), most acute leukemias are myeloid in origin in dogs. Horses can suffer equally from acute lymphoid or myeloid leukemia (Barrett et al 2017). AML is also more common in the cat and was, in many cats, caused by Feline Leukemia virus (FeLV) until the advent of FeLV vaccination which markedly decreased the frequency of these leukemias. T cell acute leukemia (which is very difficult to distinguish from lymphoma, which has heavily infiltrated the marrow and numerous tumor cells are in blood) is the next most common type we see at Cornell University, with rare B cell acute leukemias. Unlike humans, AML can occur in dogs of any age and we frequently see it in very young dogs (this seems particularly true of the T-ALL/lymphoma). Although a diagnosis of acute leukemia can be made from blood and bone marrow aspirate smears, differentiation of AML from ALL requires more than morphologic assessment of blasts (all blasts can look alike). Cytochemistry and immunophenotyping (but not clonal gene rearrangements) are required for classification of acute leukemia – ALL from AML and the different types of AML. This may be very important as ALL may be more responsive to aggressive chemotherapy and are treated with different chemotherapeutic agents (typically a lymphoma protocol) than AML. In individual cases it can be difficult to determine the type of acute leukemia. This is because neoplasms frequently display aberrant marker expression or lineage infidelity. For example, in our experience, many cases of AML can express T cell markers (CD3, weak CD5) and rarely B cell markers (CD22) (Stokol et al 2015). The expression of T cell markers in AML (especially monoblastic variants) make sense with new information being gleaned on hematopoiesis from murine models. These models posit that erythroid and megakaryocytic cells branch off before lymphoid and myeloid progenitors and that there is common lymphoid-myeloid progenitor. Indeed, B cells also appear to split off earlier from this common lymphoid-myeloid progenitor, with T cells and monocytes (in particular) staying together longer before differentiating down their specific lineages (Kawamoto et al 2010, Görgens et al 2013). In cases of acute leukemia, all available tools at our disposal (multiple markers, immunophenotyping and cytochemical staining) should be applied to determine lineage and clearly differentiate ALL from AML. In individual cases, the diagnosis is still difficult and then it comes down to preponderance of evidence – is the leukemia demonstrating more myeloid than lymphoid markers (then it is likely an AML) or lymphoid than myeloid markers (then it is likely an ALL). Most reports of acute leukemia in dogs have differentiated ALL from AML based on very limited marker expression and it is likely that many of these leukemias were misclassified.
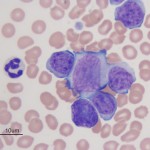
Acute lymphoid/lymphoblastic leukemia
Acute lymphoid/lymphoblastic leukemia is generally sub-classified by phenotype as T-ALL, B-ALL or NK-ALL (natural killer cell). The neoplastic cells are not invariably large. We have seen small, intermediate, and large cell variants of ALL. The WHO recently updated their classification scheme of lymphoid neoplasms (Swerdlow et al 2008).
- T-cell ALL usually expresses T cell markers, CD3 and CD5, and are usually MHCII positive. They are usually negative for CD34 (stem cell marker), CD4 (helper/regulatory T cell marker) and CD8 (cytotoxic T cell marker). They do not usually express enzymes or Sudan B black (SBB), but some can be weakly positive for leukocyte alkaline phosphatase (LAP) and focally positive for α-naphthyl butyrate esterase (ANBE).
- B-cell ALL will express B cell markers, such as CD21, CD22, CD20 and Pax-5. They may drop off expression of CD45 (common leukocyte antigen) and some may co-express CD3. They are negative for enzymes and SBB.
- NK-ALL may be negative for all markers, but can express CD8 or CD5. A single case we have seen was positive for MHCII and negative for T cell antigens and CD34. They can have red cytoplasmic granules.
Once a leukemia has been designated as lymphoid, the biggest decision to make is: Is it an immature (precursor) lymphoma from an extramedullary site infiltrating the bone marrow or circulating in blood (leukemic phase of lymphoma) or is it an ALL arising in the bone marrow? This distinction can be difficult to make and we use various bits of information gleaned from the animal (see more below). The difficulty in distinguishing lymphoid leukemia from lymphoma not only applies to immature or precursor cell lymphoid neoplasms, but also to the mature or chronic lymphoid neoplasms, i.e. mature forms of lymphoma and chronic lymphocytic leukemia (CLL). Indeed, sometimes we call these tumors lymphoma/leukemia when the distinction cannot be made with confidence. Ultimately, the differentiation of lymphoid leukemia (acute or chronic) from lymphoma (immature or mature) boils down to one question: Which is the main site of involvement? Bone marrow (for ALL), blood (for CLL) or extramedullary tissues (for lymphoma). The lymphoid neoplasms should be considered spectrums of one disease. For precursor cell neoplasms, at one end of the spectrum you have primary bone marrow involvement which dominates the clinical picture or an ALL. On the other end of the spectrum, you have a picture dominated by peripheral lymphoid tissue involvement (lymph nodes, liver, spleen), i.e. immature/lymphoblastic lymphoma. And then you have everything in between, where there is overlap between these two extremes. The animals showing overlap are the most difficult to definitively characterize.
Acute myeloid leukemia
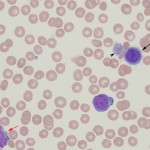
Acute myeloid leukemia in animals fit into the World Health Organization classification of AML-NOS (not otherwise specified), which are generally sub-classified according to the old French-American-British (FAB) criteria (Swerdlow et al 2008, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edition). The FAB criteria depends on morphologic features of differentiation and expression of differentiation markers by the tumor cells. In our experience, AML-NOS, acute monoblastic/mononocytic leukemia (AML-M5) is the most common subtype of AML in the dog (Stokol et al 2015, Stokol et al 2017) and acute erythroid leukemia (AML-M6) is the most common subtype seen in the cat (Blue et al 1988, Jain et al 1991), although the latter is infrequently seen nowadays since the advent of FeLV vaccination. With the advent of epigenetics and genetic testing, the classification scheme for myeloid leukemias has been recently revised and will continue to be a work in progress (Arber et al 2016). More details on AML are provided on this page.
Chronic leukemia
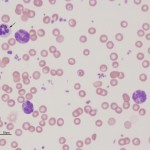
Chronic leukemias are characterized by the expansion of differentiated cells in the blood. Chronic leukemia can be lymphoid or myeloid in origin. The term “chronic” applies to the stage of maturation of the tumor, i.e. differentiated or mature. However, chronic also yields information on onset and progression. Chronic leukemia, unlike acute leukemia, usually has a protracted clinical course and these neoplasms are often diagnosed as an incidental finding in animals presenting for other complaints. Most importantly, all chronic myeloproliferative diseases (chronic myeloid leukemia) are exceedingly rare in animals. Most causes of very high leukocyte (non-lymphoid), platelet and red blood cell counts are not due to chronic myeloid leukemia but cytokine-driven or reactive extramedullary causes of bone marrow hyperplasia, e.g. paraneoplastic leukocytosis secondary to various carcinomas is more likely than a chronic neutrophilic leukemia (Dole et al 2004, Benson et al 2015), erythrocytosis secondary to hypoxia, renal disease or underlying neoplasia is more likely than a polycythemia vera (Moore and Stepien 2001, Axon et al 2008, Durno et al 2011). Bone marrow aspiration is generally not useful for diagnosis of chronic myeloid leukemias because the marrow shows hyperplasia of the affected cell lines in either neoplastic or non-neoplastic mature cell expansions, i.e. a myeloid hyperplasia is observed in both CGL and a leukocytosis that is cytokine-driven from a neoplasm. Similarly, a diagnosis of CLL should be reserved for those patients in which a cause for the lymphocytosis (e.g. reactive antigenic stimulation, epinephrine response, thymoma) is not found (Avery and Avery 2007). Immunophenotyping and clonality can also help confirm a diagnosis of CLL, but is not without caveats (see below). Just like chronic myeloid leukemias, a bone marrow aspirate may not be useful for diagnosis of CLL as the marrow is not always infiltrated (it is not the primary site of origin). Chronic lymphocytic leukemia is also uncommon in animals.
For all types of chronic leukemia, a presumptive diagnosis can be made if there is an unexplained persistent (and generally increasing) leukocytosis documented on multiple occasions (over several months). An intensive diagnostic investigation should be undertaken in such animals to rule out occult neoplasia or abscesses which may be driving the expanded cell numbers. In our experience, the most common chronic leukemia is CLL. This is also the most common cause of a persistent lymphocytosis in dogs.
Chronic lymphocytic leukemia
Chronic lymphocytic leukemia is considered a neoplasm of accumulation, i.e. the genetic mutation prevents cell death, resulting in accumulation of the mature differentiated cells. There are three different general types of CLL:
- B CLL: This is thought to arise from post-germinal B cells in the lymph nodes. These can infiltrate the bone marrow.
- T CLL: This can be of helper/regulatory (CD4) or cytotoxic (CD8). Some variants are double negative (not expressing CD4 or CD8) or rarely double positive (expressing both CD4 and CD8). In both dogs and cats, this is most common type of CLL (cytotoxic T cells in the dog and regulatory/helper T cells in the cat). The tumor is thought to arise primarily in the spleen and marrow is usually not infiltrated extensively by the neoplastic cells. The peripheral blood is characterized by a marked lymphocytosis of mostly mature small lymphocytes. Many T-CLLs are of granular lymphocyte morphology in the dog, i.e. their cytoplasm contains small red cytoplasmic granules (Vernau and Moore, 1999, McDonough and Moore, 2000).
- NK-CLL: Rare.
Since the mature lymphoid leukemias (CLL) appear to arise in extramedullary sites, they do not always involve the bone marrow so bone marrow is not always helpful for diagnosing CLL. Even though they arise in lymphoid organs, the main site of involvement is blood, helping distinguish the mature chronic lymphocytic leukemias from mature lymphomas (e.g. small cell lymphoma). Similar to immature cell lymphoid neoplasms, mature lymphoid tumors comprise a spectrum of likely one disease. At one end of the spectrum you have primary blood involvement which dominates the clinical picture or a CLL. On the other end of the spectrum, you have a picture dominated by peripheral lymphoid tissue involvement (lymph nodes, intestinal tract), i.e. small cell lymphoma. And then you have everything in between, where there is overlap between these two extremes. The animals showing overlap are the most difficult to definitively characterize.(see more below and spectrum image).
A lymphocytosis could be due to reactive antigenic stimulation (e.g. an expected finding in young dogs, cats and horses, Ehrlichia canis infection in dogs), other tumors (e.g. thymoma, uncommon), epinephrine response (cats, common, should be transient), and hypoadrenocorticism in dogs (uncommon). Clonality assessment can help distinguish between non-neoplastic and neoplastic causes of lymphocytosis, but clonal expansions of T and B cells have been documented in dogs with Ehrlichia canis and Borrelia burgdorferi infection, respectively (Avery and Avery 2007) so infectious disease testing should be done in all animals with a persistent lymphocytosis that is not explainable. Immunophenotyping can also help…expanded numbers of a single type of lymphocyte (e.g. CD4 helper cells) or aberrant expression patterns (e.g. decreased expression of MHCII) can help distinguish neoplastic from non-neoplastic lymphocytosis.
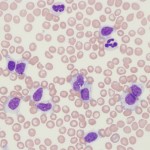
Again, some infectious agents, notable Ehrlichia canis, can cause a clonal expansion of CD8+ T cells, which may have granules in their cytoplasm (Heeb et al 2003). So clonality or a uniform phenotype does not always equal cancer.
Chronic myeloid leukemia
With chronic myeloid leukemia, the genetic mutation causes proliferation and differentiation, so these diseases are characterized by high counts of the involved cell lineage, in peripheral blood. Chronic myeloid leukemias fit under the WHO designation of chronic myeloproliferative disorders, of which there are several sub-types as indicated below (Arber et al 2016). The term “chronic myeloid leukemia” can be confusing as it is used to describe the umbrella term for these various sub-types (although in reality, the term chronic myeloproliferative disorder should be used instead), as well as a specific subtype, which is characterized by the “Philadelphia chromosome” or a translocation between the genes bcl (chromosome 22) and abr (chromosome 9) which results in constitutive cell proliferation secondary to a tyrosine kinase. A similar translocation has been identified in dogs and is called the “Raleigh” chromosome (Breen and Modiano 2008).
As for CLL, any expansion in leukocytes (non-lymphoid), platelets or red blood cells must be distinguished from cytokine-driven or reactive responses. Chronic myeloproliferative neoplasms are quite rare in animals and a cytokine-driven response should be considered the main differential diagnosis for increases in hematopoietic lineages before a chronic myeloid leukemia. Cytokine stimulation (either due to inflammation or secondary to cancer) is the most common cause of an unexplained neutrophilia or monocytosis in an animal.
The following are sub-types of chronic myeloproliferative disorders:
- Chronic myeloid leukemia: Expansion of multiple myeloid lineages, which is associated with the Philadelphia chromosome in humans. Must be distinguished from the more common cytokine-driven response, e.g. paraneoplastic leukocytosis, sequestered inflammation.
- Polycythemia vera: Chronic erythroid leukemia, primary erythrocytosis or polycythemia vera. Must be distinguished from erythropoietin-driven response (e.g. secondary to hypoxia or another tumor), which are far more common. Studies in humans have shown that most polycythemia vera patients (>90%) have a homozygous mutation in Janus kinase 2 (JAK2), which is a non-receptor tyrosine kinase downstream of the erythropoietin receptor (Levine et al 2005, Stein et al 2015). The mutation (valine to phenylalanine at amino acid 617 or V617F) causes constitutive activation of the receptor signaling, without the need for erythropoietin, which drives erythropoiesis. A similar JAK2 mutation has been identified in a dog with polycythemia vera (Beurlet et al 2011).
- Chronic granulocytic leukemia: Expansion of neutrophils (chronic neutrophilic leukemia), eosinophils (chronic eosinophilic leukemia), basophils (chronic basophilic leukemia) (Mears et al 1997, Barrs et al 2002, Gelain et al 2006). Expanded numbers of granulocytes must be distinguished from the more common cytokine-driven responses, e.g. allergies or hypersensitivity reactions, immune-mediated reactions (e.g. idiopathic hypereosinophilia in Rottweilers [Sykes et al 2001]), hypereosinophilic syndrome or gastrointestinal eosinophilic sclerosing fibroplasia in cats (McEwen et al 1985, Linton et al 2015), paraneoplastic leukocytosis (e.g. mammary carcinoma, lymphoma) (Benson et al 2015), sequestered inflammation.
- Essential thrombocythemia: Chronic platelet leukemia. Heterozygous mutations in JAK2 (the thrombopoietin receptor signaling is triggered by lower activities of JAK2 than the erythropoietin receptor) or mutations in mpl, the thrombopoietin receptor, have been identified in human patients with essential thrombocythemia (Sanchez and Ewton 2006, Tefferi 2010). Essential thrombocythemia is a very rare cause of thrombocytosis in animals. Drugs, inflammation, iron deficiency, and cancer are far more common causes (Sellon et al 1997, Rizzo et al 2007, Neel et al 2012) and are likely mediated directly or indirectly via increased thrombopoietin concentrations.
- Idiopathic myelofibrosis: The name of this type of chronic myeloproliferative disease is misleading because the leukemia is due to a megakaryocyte defect and not a fibroblast defect, i.e. the fibrosis is secondary. However, it is entrenched in the literature. This type has not been conclusively identified in animals and most reported cases of myelofibrosis are secondary to immune-mediated disease (dogs [Stokol et al 2000, Weiss and Smith 2002], feline leukemia virus-induced AML or myelodysplastic syndrome (MDS) (Blue 1998), other tumors (Weiss and Smith 2002) or bone marrow injury (drugs etc).
There are other types of chronic myeloproliferative disorders that also show features of abnormal maturation or myelodysplasia. These are categorized as a separate group of neoplasms (myelodysplastic/myeloproliferative or MDS/MPD) and include chronic myelomonocytic leukemia, atypical chronic myeloid leukemia (negative for Philadelphia chromosome [Marino et al 2017]), MDS/MPD with sideroblasts and thrombocytosis, and juvenile myelomonocytic leukemia (Arber et al 2016). There has also been a report of dendritic cell leukemia in a dog (Allison et al., 2008).
Lymphoma
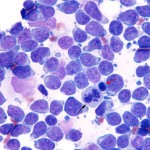
This is a neoplastic proliferation of lymphoid cells (excluding plasma cells) that arises in extramedullary tissue. Lymphoma cells can, but do not always (by morphologic criteria) infiltrate the marrow secondarily (called stage V disease). The bone marrow infiltrates are often seen amongst a normal hematopoietic population and range from patchy to diffuse. In most affected animals, particularly B cell lymphomas, the tumor cells comprise <25% of cells in marrow. Some patients with lymphoma can also have circulating lymphoma cells (with or without bone marrow involvement), which has various terms, including leukemic phase of lymphoma (used at Cornell University), lymphoma with circulating cells, lymphoma-associated leukemia, lymphoma with secondary leukemia (and is also classified as stage V disease). A lymphoma with a leukemic phase is more commonly seen in dogs than cats, but does occur in cats with thymic, hepatic, and splenic lymphoma. Also, animals can have marrow infiltrates without detectable circulating lymphoma cells (e.g. spinal lymphomas in cats).
Lymphoma cells in blood or bone marrow will resemble the primary tumor, i.e. will be blasts if the primary tumor is a large cell lymphoma or intermediate cells from an intermediate cell lymphoma. Blasts from large cell lymphomas are easier to identify in peripheral blood than lymphoma cells from intermediate or small lymphomas. This is because blasts are not normally seen in blood, whereas normal blood lymphocytes consist of small, with fewer intermediate, cells. The number of circulating lymphoma cells varies from very low (i.e., not included in the differential cell count) to very high. When there are large numbers of lymphoid cells in blood or bone marrow (>25% or diffuse infiltrates), it can be very difficult to distinguish between lymphoma and leukemia, i.e. a lymphocytosis consisting of small lymphocytes could be a small cell lymphoma with a leukemic phase or a CLL; a lymphocytosis of larger cells could be a large cell lymphoma with a leukemic phase or an acute leukemia (do not assume it is lymphoid until phenotyping is done).
Lymphoma or leukemia?
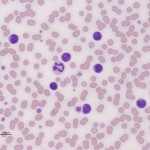
A large cell lymphoma with a leukemic phase can mimic an acute leukemia (myeloid or lymphoid), which is characterized by variable numbers of blasts in circulation. Alternatively, a small to intermediate cell lymphoma with a leukemic phase can resemble a CLL or an ALL (which can consist of small to intermediate cells usually with fine chromatin, versus AML which usually consists of blasts). We use the following to distinguish between a lymphoma with a leukemic phase and an acute leukemia (which arises primarily in the bone marrow) before we know the true phenotype of the cells (see also the algorithm above):
- Cytopenias: The absence of moderate to severe cytopenias (non-regenerative anemia, thrombocytopenia, neutropenia) in peripheral blood in lymphoma versus acute leukemia (ALL or AML). Cytopenias, if present, are mild (usually non-regenerative anemia) and related to inflammatory disease in lymphoma. In contrast, acute leukemias frequently efface the bone marrow resulting in peripheral cytopenias (usually multiple cell lines affected) that are moderate to severe. In our experience, a moderate to severe thrombocytopenia and a mild non-regenerative anemia are the most common hematologic findings in dogs with T-ALL. Some dogs and horses with lymphoma (usually T cell) can show similar cytopenias or may have a solitary unexplained neutropenia.
- Marrow infiltrates: In most patients with lymphoma, no or low numbers of infiltrating lymphoma cells can be identified in bone marrow aspirates. In some patients, more extensive infiltrates can be seen, but these infiltrates are usually focal and comprise <25% of total marrow cells (the WHO criterion for distinguishing between an acute lymphoblastic leukemia and precursor B lymphoma, which are variants of the same disorder [Swerdlow et al 2008]). In rare cases, particularly with T cell neoplasms in the dog, neoplastic T cells (which are not always blasts, they are frequently small to intermediate in size but have finer chromatin than mature lymphocytes) can extensively infiltrate the marrow (>25% of cells) and, if there are concurrent cytopenias in peripheral blood, these neoplasms are difficult to impossible to distinguish from an ALL by bone marrow aspirate alone (see June Case of the Month 2016). The distinction between these lymphoid neoplasms may be academic because they are treated with the same agents (currently anyhow), however lymphoma may respond more favorably to chemotherapy than ALL. Ultimately, the distinction between lymphoma versus ALL essentially comes down to the main site of involvement, i.e. bone marrow = ALL; extramedullary tissues = lymphoma. The same concept applies to a small cell lymphoma and CLL (see above, although for the latter, the main site of involvement = blood not bone marrow).
- Extramedullary tissue involvement: Since lymphoma arises in extramedullary tissue, organ involvement is substantial resulting in moderate to marked organomegaly. Note that both acute and chronic leukemias can also infiltrate extramedullary tissue (lymph nodes and spleen, particularly), but the organomegaly is usually milder than a primary lymphoma arising in the affected tissue (there are always exceptions to this). On histopathologic examination, a vascular orientation for leukemic cells (e.g. hepatic sinusoids or periportal area in liver biopsies) would support a primary leukemia versus lymphoma, however we have seen acute myeloid leukemia form more diffuse and even nodular infiltrates, resembling masses (Barrett et al 2017, Stokol et al 2017 Frontiers in Veterinary Science doi: 10.3389/fvets.2017.00076).
- Stem cell marker expression: Lymphomas are generally negative for the stem cell marker, CD34. We have seen a few cases of B cell lymphomas (which are usually large cell lymphomas) that are positive for CD34. Most AML are positive for CD34 and CD45 (common leukocyte antigen) and are MHCII-negative (Stokol et al 2015 and 2017), but the majority of ALL are negative for CD34. The latter are usually positive for MHCII, which may be a useful distinguishing feature and may drop off CD45, but neither of the latter findings are specific for ALL vs AML. Chronic leukemia (any lineage) are not expected to be positive for CD34 because they represent more differentiated cells (not immature blasts).
Myelodysplastic syndromes
Myelodysplastic syndromes (MDS) are a group of clonal hematologic disorders that share the general characteristics of ineffective hematopoiesis and abnormal maturation in more that one cell line. Because MDS can progress to acute myeloid leukemia (AML), they have been called preleukemia. Subgroups of MDS are defined by specific criteria but, in general, typical hematologic findings are cytopenias in peripheral blood with normo- to hypercellular marrow, a fairly heterogeneous mixture of hematopoietic cells in marrow, and morphologic evidence of abnormal maturation. In the erythroid series, morphologic abnormalities include asynchronous maturation of nucleus and cytoplasm, megaloblastic cells, and siderotic granules. Atypia in megakaryocytes includes very small (about the size of a neutrophil) mononuclear and multinuclear megakaryocytes and larger megakaryocytes with lumpy round nuclei or nuclei with bizarre lobulation. In the granulocyte lines, abnormalities include hyposegmentation, hypersegmentation,abnormal granules, and an increase in immature cells. In some forms of MDS, the percentage of myelo- and/or monoblasts is increased (>5% but < 30%). Identifying the morphologic evidence of dysplastic maturation is important to distinguish neoplastic ineffective hematopoiesis from that caused by immune mechanisms.
A classification system developed for human patients by the French-American-British (FAB) cooperative group is applicable, with modifications, to veterinary patients.
The natural progression of MDS is variable. The outcome may be death due to lack of mature blood cells (severe anemia, thrombocytopenia, neutropenia), stable disease and death due to unrelated causes, or progression to acute myeloid leukemia and infiltration of extramedullary tissue by non-lymphoid blasts. At present, there is no effective therapy for MDS in animals but supportive treatment, especially transfusions, can prolong survival and maintain quality of life.
