Interpretation
Bile peritonitis
Explanation
Question 1: The increases in ALT and AST activity indicates hepatocellular injury. The increase in AST activity can also be partially attributed to muscle injury, as supported by the increase in creatine kinase activity (albeit mild). There is evidence of cholestasis as indicated by the increases in ALP and GGT activities, and bilirubin concentrations (total and direct). In addition to cholestasis, the increase in GGT also may be due to biliary hyperplasia. The increase in ALP activity can also be attributed to stress resulting in an increase in the corticosteroid-induced ALP isoform, corroborated by the concurrent neutrophilia and lymphopenia on the CBC. The low iron concentration may be due to inflammation (sequestration due to inflammatory cytokines) or physiologic diurnal variation. Established inflammation is supported the leukocytosis, mature neutrophilia, and monocytosis (the neutrophil count is too high to attribute to a corticosteroid alone). The mildly decreased potassium concentration could be attributed to gastrointestinal losses with vomiting. The mildly decreased creatinine concentration is suggestive of decreased muscle mass, which is plausible given the patient’s small breed size.
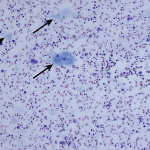
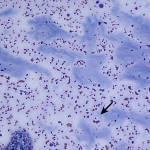
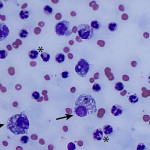
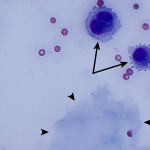
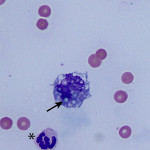
The increased total protein concentration (>2.5 g/dL) and nucleated cell count (>7,000/ul) with predominance of neutrophils (81%) indicate an exudative effusion (Question 2). The remaining leukocytes consisted of 18% macrophages and 1% small lymphocytes (lymphocytes not pictured). The neutrophils were non-degenerate arguing against, but not excluding, a septic (infectious) process. In addition, no bacterial or other infectious agents are observed. There was a large amount of extracellular pale blue amorphous material, in clumps or lakes, in the background (Figure 1), some of which was also present within macrophages (Figure 4). This material is the key to the underlying cause of the effusion.
Question 3: The two main differential diagnoses for the pale blue amorphous material in this sample are mucin or fibrin, which appear similar cytologically. Fibrinous peritonitis is a result of chronic peritonitis that causes exudation of large amounts of fibrinogen and subsequent formation of fibrin, which results in fibrinous/fibrous adhesions. This is a more common phenomenon in large than small animals. However, an uncommon syndrome termed “sclerosing encapsulating peritonitis” has been reported in small animals, which can be idiopathic or secondary to foreign body ingestion, bacterial peritonitis, steatitis, and other conditions.1 This syndrome is also referred to as “abdominal cocoon syndrome” in human beings, because abdominal organs become enveloped in the fibrous tissue.1 Mucin could be present due to a secretory epithelial tumor (e.g. mucinous adenocarcinoma), a myxosarcoma12, or “white bile,” seen as a sequela to extrahepatic biliary obstruction.2,3 The term “white bile” is somewhat of a misnomer, as it is colorless and devoid of all bile constituents except mucinous material.3 The chemistry abnormalities indicate hepatobiliary disease making leakage of “white bile” into the peritoneal cavity the leading differential diagnosis. To confirm this presumptive diagnosis, an abdominal ultrasound examination and measurement of peritoneal fluid bilirubin to compare with the serum bilirubin are recommended (Question 4).
Additional diagnostics
The patient was taken for an abdominal ultrasound examination. The gallbladder was moderately enlarged and almost 100% filled with structured, mostly hypoechoic material that contained innumerable radial, linear hyperechogenicities and a hyperechoic rim. A small section of the gallbladder wall was indistinct and communicated with a local accumulation of moderately echoic fluid. The surrounding mesentery was also severely hyperechoic. A short segment of the bile duct was moderately dilated and contained anechoic material (compatible with bile or mucus). The peritoneal cavity contained a small to moderate volume of moderately echoic fluid and the mesentery was diffusely moderately to severely hyperechoic in the right cranial abdomen. These findings were diagnostic for a ruptured gallbladder mucocele, with resulting bile peritonitis.
Out of academic interest, the peritoneal fluid bilirubin was measured in the clinical pathology laboratory, and compared to that of serum bilirubin. The peritoneal fluid total bilirubin was 1.3 mg/dL, which was 3.3 times greater than the serum bilirubin (0.4 mg/dL) confirming a bile peritonitis. Peritoneal fluid bilirubin at least 2 times greater than serum bilirubin is considered diagnostic for a bile peritonitis, in most cases.4,5
Discussion
A gallbladder mucocele is a dilation of the gallbladder with accumulated mucoid secretion.6 Gallbladder mucoceles are thought to have been rare in dogs prior to the early 1990s, but are now considered a common cause of extrahepatic biliary disease in this species.7 Shetland Sheepdogs and Cocker Spaniels are overrepresented breeds.7–9 Commonly reported clinical signs and physical examination findings include vomiting, anorexia, lethargy, abdominal pain, icterus, tachypnea.7 The most common chemistry abnormalities include increased ALT, AST, ALP, and GGT activities and high concentrations of bilirubin, as was present in this case.7 Dogs with ruptured gallbladder mucoceles had significantly higher mean serum activities of ALP and AST, mean serum total bilirubin concentrations, and mean total WBC concentrations, as compared to dogs with intact gallbladders, in one study.7
Histologically the lesion is characterized by hyperplasia of the mucus-secreting mucosal glands.6 The underlying cause of this hyperplastic response and gallbladder mucocele formation is unknown.6,9 Accepted inciting causes in human medicine include infiltrative disease of the cystic duct or cholelithiasis causing cystic duct obstruction, however dogs with gallbladder mucoceles do not show evidence of these concurrent abnormalities.7 Dyslipidemia may play a role in the disease pathogenesis, as an association has been documented in Shetland Sheepdogs with gallbladder mucoceles.8 New research suggests a significant increase in the production of a particular gel-forming mucin (Muc5ac) in gallbladder secretions from dogs with mucoceles resulting in an abnormal ratio of this mucin to the normally predominant Muc5b.9 This research also reports significant increases in 24 proteins, including IgA, IgG, complement factors, and protease inhibitors, in canine mucocele samples.9 Therefore, it is likely that gallbladder mucoceles result from not only hypersecretion, but also dysfunction of mucus-secreting cells within the gallbladder mucosa.7,9 The secreted mucus can extend into the cystic, hepatic, and common bile ducts, resulting in variable degrees of extrahepatic biliary obstruction.6 With progressive accumulation of semisolid mucus in the gallbladder lumen, the gallbladder wall is at risk of ischemic necrosis, rupture, and release of mucoid contents into the peritoneal cavity, as seen in this case.6
Bile can be seen in a number of different sample types and has varying cytologic appearances. Most commonly, bile is seen in the form of casts in liver aspirates as dark green linear striations delineating hepatocytes indicating cholestasis (see Cholestasis page or Liver Atlas). Normal bile, uncommonly sampled by fine needle aspiration of the gallbladder, is acellular and appears as amorphous to wispy, lavender to blue-green material on gray-brown precipitate on Wright’s stained cytologic smears.10-11 The presence of bile in peritoneal fluid samples is evidence of gallbladder or bile duct rupture, and is considered a surgical emergency.7 Bile is an irritating substance resulting in an inflammatory response and a chemical peritonitis, which is typically exudative.4,5 In addition to gallbladder mucoceles, rupture of the biliary system can occur secondary to bile duct obstruction (e.g. cholethiasis), trauma (e.g. gunshot wounds, gastric dilatation and volvulus), and iatrogenic (e.g. fine needle aspiration or percutaneous biopsy).4,5 Most commonly in bile peritonitis, bile appears as yellow or green to blue-black granular material seen extracellularly, as well as in the cytoplasm of macrophages or neutrophils.4,5 Grossly, the sampled peritoneal fluid can appear brown, yellow, or green. The current case is an example of the blue amorphous extracellular material, commonly referred to as “white bile,” which is an alternative means of cytologically diagnosing a bile peritonitis.2-5
In the primary veterinary literature, “white bile” has only been reported in cases of dogs with rents in the common bile ducts,2 however anecdotally it has been more commonly associated with rupture of gall bladder mucoceles, as in this case (personal communication Drs. E. Behling-Kelly and R. Allison). In the published case series and the current case, no typical bile pigment or bilirubin crystals were observed on cytologic examination.2 This material is negative for Hall’s bile stain, and stains positive with Alcian blue and mucicarmine, indicative of mucin.2 The reason why this material lacks bile pigments or bilirubin is not completely understood. It has been experimentally produced in dogs after ligation of both the common bile duct and cystic duct resulting in reverse flow of bile from the extrahepatic ducts into the liver.3 In this same study, dark green (or “black”) bile occurred only when the common bile duct was ligated and the flow was from the extrahepatic ducts into the gallbladder.3 Therefore, it is interpreted to be a sequela of extrahepatic biliary obstruction, cholestasis, “back wash” of biliary secretions into the liver, hepatic lymph, and venous blood, and biliary epithelial secretion of clear bile-free mucoid material.2,3
Peritoneal fluid analysis with cytologic evaluation is a useful adjunctive tool in the diagnosis of bile peritonitis and can be evaluated on an emergency basis to help guide further diagnostic tests and therapy. The presence of yellow-green or blue-black granular material or light blue amorphous fibrillar material in a cytologic smear of peritoneal fluid should raise the index of suspicion for a bile peritonitis, especially in a patient with biochemical evidence of hepatobiliary disease. Note, that we have seen some cases of bile peritonitis, where bilirubin concentrations in the peritoneal fluid are not higher than serum concentrations (personal communication, Dr. T Stokol), thus the 2x increase in total bilirubin in peritoneal fluid versus serum should not be relied upon to confirm a bile peritonitis in cases such as this one.
Case follow-up
In light of the cytologic and abdominal ultrasound findings, the patient was taken to emergency surgery. A cholecystectomy was performed, samples of bile and peritoneal fluid were submitted for bacterial culture, and samples of gallbladder and liver were submitted for histopathologic examination. There was no growth on aerobic or anaerobic culture for both the bile and peritoneal fluid. Histopathologic examination of resected tissue confirmed the presence of a gallbladder mucocele with focal rupture. The remaining histologic findings in the biliary tree and liver were considered secondary to the gallbladder mucocele. At the last client communication (2 months after surgery), the patient was doing well at home. The liver enzyme activities had trended downward since surgery, but remained mildly increased, as has been reported previously.7
References
- Veiga-Parga T, Hecht S, Craig L. Imaging Diagnosis–Sclerosing Encapsulating Peritonitis in a Dog. Vet Radiol Ultrasound [Internet]. 2015;56(6):E65–9.
- Owens SD, Gossett R, McElhaney MR, Christopher MM, Shelly SM. Three cases of canine bile peritonitis with mucinous material in abdominal fluid as the prominent cytologic finding. Vet Clin Pathol. 2003;32(3):114–20.
- Hashmonai M, Kam I, Schramek A. The Etiology of “ White Bile ” in the Biliary Tree. J Surg Res. 1984;486(1984):479–86.
- Valenciano A, Arndt T, Rizzi T. Effusions: abdominal, thoracic, and pericardial. In: Valenciano A, Cowell R, editors. Diagnostic cytology and hematology of the dog and cat. 4th ed. St. Louis: Elsevier; 2014. p. 257–8.
- Thompson C, Rebar A. Body cavity fluids. In: Raskin R, Meyer D, editors. Canine and feline cytology: a color atlas and interpretation guide. 3rd ed. St. Louis: Elsevier; 2016. p. 202–3.
- Stalker M, Hayes M. Liver and biliary system. In: Maxie M, editor. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Fifth. Philadelphia: Saunders Elsevier; 2007. p. 383.
- Pike FS, Berg J, King NW, Penninck DG, Webster CRL. Gallbladder mucocele in dogs: 30 cases (2000-2002). J Am Vet Med Assoc. 2004;224(10):1615–22.
- Aguirre AL, Center S a, Randolph JF, Yeager AE, Keegan AM, Harvey HJ, et al. Gallbladder disease in Shetland Sheepdogs: 38 cases (1995-2005). J Am Vet Med Assoc. 2007;231(1):79–88.
- Kesimer M, Cullen J, Cao R, Radicioni G, Mathews KG, Seiler G, et al. Excess secretion of gel-forming mucins and associated innate defense proteins with defective mucin un-packaging underpin gallbladder mucocele formation in dogs. PLoS One. 2015;10(9):1–20.
- Flatland B. If you have the gall . . . Vet Clin Pathol. 2009;38(3):280.
- Peters LM, Glanemann B, Garden OA, Szladovitz B. Cytological findings of 140 bile samples from dogs and cats and associated clinical pathological data. J Vet Intern Med. 2016; 30:123-131.
- Riegel CM, Stockham SL, Patton KM, Thomas CL. What is your diagnosis? Muculent pleural effusion from a dog. Vet Clin Pathol. 2008, 37(3):353-356.
Authored by: Dr. A. Newman (Clinical Pathology senior resident). The author would like to thank Dr. Mason Jager (Anatomic Pathology resident) for providing the histopathologic images for this case.