Interpretation
Boid Inclusion Body Disease; Marked lymphocytosis; Mild heterophil toxicity
Explanation
Hematology testing and evaluation of non-mammalian blood smears can be challenging for several reasons. All blood cells are nucleated, including red blood cells and platelets, so automated hematology analyzers are not routinely used for white blood cell counts, as these analyzers principally rely on detection of nuclei to count white blood cells. Instead, white blood cell counts in non-mammalian species are generally performed manually with a hemocytometer and one of two specific staining protocols, Natt-Herrick stain or phloxine-B stain.2
Additionally, when evaluating blood smears from these species, the hematologist must be able to confidently distinguish heterophils (the neutrophil equivalent) from eosinophils, understand species differences in heterophil nuclear shape, and distinguish lymphocytes from thrombocytes (Question 1)2. Both heterophils and eosinophils have numerous pink cytoplasmic granules, but the granules are of different shape, and may also be a slightly different shade of pink. Eosinophil granules are typically round, while heterophil granules can be elongated or fusiform in shape. In birds and lizards, the heterophil nucleus is lobulated, similar to the neutrophil nucleus in a mammal. However, in snakes, turtles, and tortoises, the heterophil nucleus is normally round or oval. This can make it difficult to identify a heterophilic left shift, so inflammatory leukograms in these reptiles are typically identified when there are toxic changes in heterophils. Inflammation may sometimes also cause heterophils in species which are normally not lobulated to become more lobulated.
Lastly, lymphocytes must be distinguished from thrombocytes. Lymphocytes are similar in appearance to those seen in mammals, with a high nuclear:cytoplasmic ratio, clumped chromatin, and a small amount of clear or light blue cytoplasm. Thrombocytes can be of similar size to lymphocytes, and can also have a fairly high nuclear:cytoplasmic ratio. However, they often have an even more condensed and dark nucleus than lymphocytes, and in some species, particularly reptiles, can be elongated rather than round. Additionally, they commonly can have a few small vacuoles or granules within the cytoplasm often adjacent to one end of the nucleus.
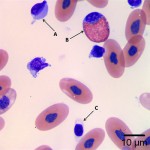
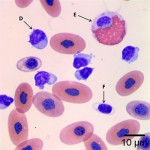
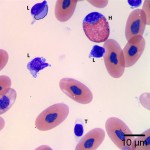
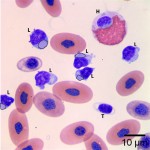
Using these characteristics, the labeled blood cells in Figures 1a and 2a are identified as lymphocytes (A and D), heterophils (B and E), and thrombocytes (C and F) (Question 2). Compared to heterophil B (a normal heterophil), heterophil E has several small irregular blue inclusions within the cytoplasm, which are most easily identifiable in the area of the cytoplasm that does not contain granules. These inclusions are Döhle bodies, and are indicative of mild toxic change within the heterophils (Question 4).
Within moderate numbers of the lymphocytes are oval, light-blue, smooth, variably-sized, cytoplasmic viral inclusions, which are outlined in black on Figures 1b and 2b (Question 3). These are the typical appearance on Wright’s stained smears for the viral inclusions associated with Boid Inclusion Body Disease. These inclusions are easier to identify when smears are stained with Hematoxylin and Eosin (H&E), where the inclusions appear bright pink. Because the inclusions were identifiable using Wright’s stain in this case, H&E staining was not performed
Discussion
Boid Inclusion body disease (BIBD) is a viral disease seen in boid snakes (boas and pythons) that causes characteristic cytoplasmic inclusions in many cell types. It has a worldwide distribution in captive snake collections. The disease was originally thought to be caused by a retrovirus,3 but more recent evidence points to a novel arenavirus.4 The disease can spread rapidly within colonies. The method of transmission has not been proven, but may involve the blood-sucking snake mite Ophionyssus natricis as a vector.3,5 Clinical signs can be quite variable and in boas can commonly include subclinical infections (for more than a year in some cases), intermittent regurgitation, chronic anorexia and weight loss, respiratory signs or stomatitis due to secondary bacterial infections, and various neurologic signs. The disease in pythons tends to be more severe and rapidly progressing, often with acute onset of neurologic signs.3,5
Diagnosis of the disease relies on identification of the characteristic viral inclusions, which stain brightly eosinophilic on H&E stain or light-blue on Wright’s stain. Antemortem diagnosis can be challenging, and confirmation of the diagnosis is often made histopathologically on necropsy. Clinical suspicion of the disease is based on clinical signs, exposure of the snake to other boids, and a thorough evaluation to rule out other causes of weight loss, respiratory signs, or neurologic signs. CBC findings are variable, and may be normal. In some cases, infected snakes may have a marked lymphocytosis in peripheral blood (>30,000/μl), or in the advanced stages of the disease can develop leukopenia. Viral inclusions may be seen on peripheral blood smears stained with Wright’s stain, but the sensitivity for detection is low, so the absence of viral inclusions cannot be used to rule out the disease. Detection may be enhanced by evaluation of buffy coat smears stained with H&E. Histopathologic evaluation of skin, liver, or gastrointestinal tract biopsies may have the best antemortem sensitivity.3 As with blood smear evaluation, the absence of viral particles cannot be used to rule out disease. However, sensitivity can be increased by collection of multiple biopsy specimens.5
There is no known treatment for BIBD. Supportive care (e.g. force-feeding, fluid administration, and treatment of secondary bacterial infections) may improve clinical signs, but the disease is invariably fatal.3,5 Gross findings on necropsy are generally related to secondary bacterial infections, such as granulomas in internal organs (e.g. liver, spleen), pneumonia, or stomatitis. Histologically, cytoplasmic inclusions can be seen in many tissues, especially hepatocytes, epithelial cells of the pancreas, kidney and gastrointestinal tract, and in the brain.5 When neurologic signs are present, the snakes often have a non-suppurative meningoencephalitis with neuronal degeneration and perivascular cuffing.3
Management to control spread to other snakes should be emphasized over symptomatic treatment of individual snakes.5 The presence of long-term asymptomatic carriers in boas can represent challenges for disease control. Since the disease is more severe in pythons, boas should be kept strictly separated from pythons in collections. Quarantine of newly-acquired boids for at least 6 months is recommended, with thorough physical exams, fecal exams, and blood work evaluations at the beginning and end of the quarantine period.5 Treatment for possible mite infestations may also be worthwhile.3 Sick snakes should be removed from the collection, and confirmed carriers of BIBD should be euthanized. The cage and cage components should be discarded or thoroughly disinfected with sodium hypochlorite or chlorhexidine.5
Case follow up
No follow-up is available for this patient, but the prognosis is poor as there is no specific treatment available for this disease.
References
- Diethelm G and Stein G. Hematologic and Blood Chemistry Values in Reptiles, Chapter 88. In: Mader D, ed. Reptile Medicine and Surgery. 2nd ed. St. Louis, MO: Saunders Elsevier, 2006.
- Nardini G, Leopardi S, Bielli M. Clinical hematology in reptilian species. Vet Clin Exot Anim, 2013; 16:1-30.
- Vancraeynest D, Pasmans F, Martel A, et al. Inclusion body disease in snakes: a review and description of three cases in boa constrictors in Belgium. Vet Rec, 2006; 158:757-761.
- Hetzel U, Sironen T, Laurinmaki P, et al. Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J Virol, 2013; 87:10918-10935.
- Schumacher J. Inclusion Body Disease Virus, Chapter 60. In: Mader D, ed. Reptile Medicine and Surgery. 2nd ed. St. Louis, MO: Saunders Elsevier, 2006.