Interpretation
Suspect malignant neoplasia of epithelial origin (squamous cell carcinoma) with moderate suppurative inflammation
Explanation
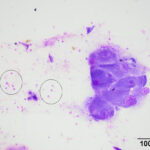
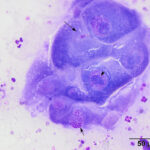
The smear of the aspirate was moderately cellular and composed of moderate numbers of non-degenerate to mildly degenerate neutrophils with a few individualized and small clusters of very large polygonal cells. There was a small amount of blood and a mixture of cellular debris, with necrotic cells, in the background. The polygonal cells had a low to moderate nuclear to cytoplasmic ratio (N:C) and mid-blue smooth to stippled cytoplasm. Their eccentric to paracentral nuclei were round with dispersed reticular to ropey chromatin and a single, mostly large, prominent nucleolus. Moderate anisocytosis and anisokaryosis were noted and neutrophils were seen within the cytoplasm of the cells. The cohesive nature of the polygonal cells supported an epithelial origin and their large size and amount and color of cytoplasm were similar to squamous epithelial cells. Based on these results, a malignant neoplasm of epithelial origin (likely squamous cell carcinoma) was suspected, however a dysplastic epithelial population secondary to the suppurative inflammation could not be ruled out (Question 2). Generic differential diagnoses for the mandibular swelling include, but are not limited to, an abscess (e.g., bite wound), inflammation (e.g., penetrating foreign body, parasitic infection, trauma), or cancer (Question 1).
|
|
|
Further diagnostic tests
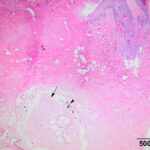
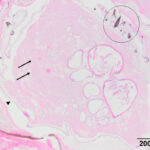
The mass and enlarged left mandibular lymph node were surgically removed and submitted for histopathologic examination. On histologic examination, the mass consisted of haired skin with an ulcerative central lesion, comprised of necrotic eosinophilic cellular debris with a moderate infiltration of neutrophils. Within the necrotic dermis, just below the surface ulceration, there was a large oval structure with irregular margins that was surrounded by a thin cuticle. Low numbers of brown round to oval projections that rarely formed a rounded point at the most distant end (spines) were attached to the cuticle. The structure was composed of several lobular formations created by large eosinophilic, cells with low N:C, similar to those seen in the cytologic smear (Figures 3 and 4). The histologic features of the structure were consistent with an arthropod larva and the overall appearance was consistent with Cuterebra spp. The submandibular lymph node was hyperplastic.
|
|
|
Discussion
The larvae of the large bee-like rodent or rabbit bot fly, Cuterebra, requires a living host for reproduction. The flies lay their eggs in the environment, typically near openings of nests and burrows or along pathways traveled by their preferred mammalian hosts. Cats become inadvertently infected with the larva as accidental hosts, when they contact the eggs, which hatch when exposed to high body temperatures. The infection usually occurs in summer or fall months in the USA. Cats are a common accidental host, versus humans or dogs, because of their outdoor feral nature and propensity to be near or around the burrows of their prey. The first instar larvae can penetrate mucosa or skin, potentially through small breaks (e.g., associated with grooming). When penetrating the skin, the larva forms a nodular lesion in subcutaneous tissue, called a warble (which usually has an opening at the surface), and feeds on host tissues, molting into second and third instars. The third instar larva extrudes itself through the surface pore and pupates in the soil, finally emerging as a mature fly.1
The infection of living mammalian tissues by larvae of flies is called myiasis and cutaneous myiasis is the most commonly seen form of myiasis. In general, cutaneous myiasis can be divided into two categories based on clinical manifestations: Furuncular and migratory myiasis.1,2 Traumatic or wound myiasis is considered a third cutaneous category by some authors1 and involves infection of a wound by flies, with screwworm flies (Cochliomyia, Chrysomya) being the worst offender. Furuncular cutaneous myiasis is usually caused by Cuterebra and other flies, such as Dermatobia (human bot fly) and Wohfharhtia (spotted flesh fly), and consists of solitary or multiple nodules or warbles. A single nodule can contain more than 1 larva. Migratory or “creeping” myiasis occurs when the larvae randomly migrate through subcutaneous tissues through tunnels, with the size of the tunnels, speed of movement, and pattern of tunnel formation dictating the cutaneous manifestation. Horse and cattle bot flies, and Hypoderma, respectively, are associated with migratory myiasis.1,2 With cutaneous furuncular myiasis, the larvae are usually confined to the created warble, however there are reports of visceral migrating larvae and Cuterebra larvae have been reported within the trachea, nasal cavity, eyes, and central nervous system in cats and dogs.3-5 When the larva are found in internal organs, it is called cavitory myiasis.2 Visceral migration in dogs and cats can be associated with severe clinical illness, including initiation of disseminated intravascular coagulation, more so in dogs and cats.3 Veterinary pathologists and neurologists at Cornell University were the first to propose that the neurologic condition known as feline ischemic encephalopathy (a neurologic syndrome in typically older outdoor cats consisting of a sudden onset of seizures, behavior changes, such as depression or aggression, or blindness in summer and fall months) was caused by the aberrant migration and potential release of toxins by Cuterebra larva.4 A second report investigating neurologic cuterebriasis in cats further supported this proposal and emphasized the connection between respiratory clinical signs and neurologic larval migration, suggesting that larva entered through the cribriform plate.5 Animals with neurologic myiasis do not always have concurrent cutaneous symptoms.
Diagnosis of cutaneous cuterebriasis is usually accomplished by visualization of the larva, which can intermittently protrude from the open pore of the warble for respiration.2 If the larva dies within the warble, the opening can seal over and appear as a solitary mass. The degrading larva can be irritating to the skin and incite inflammation and fibrosis.2 Deliberate aspiration of larva is not usually attempted due to the potential risk of larval rupture yielding a subsequent inflammatory or hypersensitivity response.3 Treatment usually involves careful extraction of the larva, while avoiding rupture, and surgical excision. A Ivermectin can be administered to kill the larva, along with dexamethasone and diphenhydramine, to reduce a hypersensitivity reaction secondary to larval death.3,5 Antibiotics have also been used to eliminate the potential for bacterial spread by the migrating larva.5
This particular case was the subject of a previously published case report in 1986.6 There has been a second reported case of cutaneous cuterebral myiasis in a cat, with the diagnoses being made from an aspirate of a subcutaneous temporal mass. 7 In the latter case, the diagnosis was based on the presence of characteristic single-pointed brown spines8 attached to fragments of cuticle. There was concurrent neutrophilic and eosinophilic inflammation. Cells observed in the case herein were not described in the latter report. These cases illustrate that, although uncommon, cutaneous myiasis should be on the differential diagnosis for a subcutaneous mass arising in an outdoor cat in summer or fall months.
References
- Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49(10):1092-1098. doi: 10.1111/j.1365-4632.2010.04577.x.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25(1):79-105. doi:10.1128/CMR.00010-11.
- Rutland BE, Byl KM, Hydeskov HB, Miniter B, Johnson CA. Systemic manifestations of Cuterebra infection in dogs and cats: 42 cases (2000–2014). J Am Vet Med Assoc. 2017;251(12):1432-1438. doi: 10.2460/javma.251.12.1432.
- Williams KJ, Summers BA, Lahunta A de. Cerebrospinal cuterebriasis in cats and its association with feline ischemic encephalopathy. Vet Pathol. 1998;35(5):330-343. doi: 10.1177/030098589803500502.
- Glass EN, Cometta AM, DeLahunta Ai, Center SA, Kent M. Clinical and clinicopathologic features in 11 cats with cuterebra larvae myiasis of the central nervous system. J Vet Intern Med. 1998;12(5):365-368. doi: 10.1111/j.1939-1676.1998.tb02136.x.
- French TW, Blue JT. What Is Your Diagnosis? Vet Clin Pathol. 1986;15(4):18-19.
- Bau-Gaudreault L, Overvelde S, Martin D. What is your diagnosis? Subcutaneous temporal mass from a cat. Vet Clin Pathol. 2018;47(2):324-325. doi: 10.1111/vcp.12601.
- Slansky F, Huckabee J. First records of rodent-infesting Cuterebra bot flies parasitizing raccoons (Procyon lotor) in North America. J Parasitol. 2006;92(6):1369-1373. doi:10.1645/GE-3567RN.1.
Authored by: S Chu (resident) and edited by T Stokol.