Cytologic findings
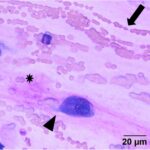
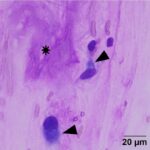
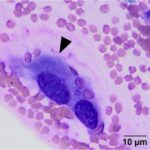
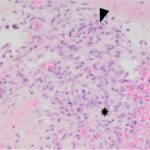
The submitted smears were moderately cellular and contained low numbers of spindle cells embedded in large amounts of long light extracellular pink streaks of material (mucinous matrix, presumptive, Question 1, Figure 1a-4a). There were low numbers of leukocytes and a small amount of blood. The spindle cells were arranged individually (Question 3) and had a moderate amount of medium to rarely dark blue cytoplasm, with some cells containing low to moderate numbers of red cytoplasmic granules. The cells had central round to oval nuclei with coarse chromatin and 1-2 small nucleoli. Several cells were binucleated or multinucleated and they displayed moderate anisocytosis and anisokaryosis (Figures 1a-4a). Red blood cells were noted to be lining up in rows, or “windrows”, in the smear (Question 3). This suggested a degree of viscosity in the sample, such as that seen with mucus (Figure 2a).
Interpretation
Myxoid-producing tumor, with differential diagnoses including a myxoma or myxosarcoma (Question 1).
Additional results
To confirm the specific tumor type and evaluate for malignant potential, an excisional biopsy was performed. A 1.6 x 1.2 x 0.8 cm piece of haired skin containing a raised, non-haired mass measuring 0.8 x 0.7 cm was submitted for histopathologic examination.
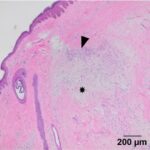
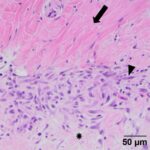
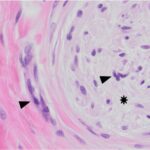
Examination of hematoxylin & eosin (H&E)-stained smears of a section of the formalin-fixed paraffin-embedded tissue revealed a fairly well-demarcated, unencapsulated, poorly cellular, expansile mass obscuring adnexal units and elevating the overlying epidermis. The mass was composed of a population of spindle cells arranged in interlacing bundles, supported by abundant amounts of a variably collagenous to myxomatous matrix. The neoplastic cells had indistinct borders, a moderate amount of eosinophilic cytoplasm, and oval to stellate nuclei, with finely stippled chromatin and a single distinct nucleolus. Scattered throughout the mass, and embedded in the matrix material, there were low numbers of inflammatory cells (neutrophils, lymphocytes, plasma cells and rare eosinophils). A focal region within the superficial dermis contained a population of reactive fibroblasts with hypertrophied nuclei, interspersed with extravasated erythrocytes (hemorrhage) and small numbers of neutrophils and lymphocytes. Anisocytosis and anisokaryosis of the neoplastic population was mild, with mitotic activity being restricted to the reactive fibroblasts (Figures 5-8). The surgical margins showed complete excision of the mass. The final diagnosis was a myxofibroma, based on the combination of mucinous and collagenous stroma within the tumor and minimal atypia or mitoses. The mild associated inflammation was attributed to previous trauma to the area. The trauma was likely just a result of every day goat life (bumped on feeder, rubbed on a fence post, etc.
Discussion
Myxofibromas are benign neoplasms of fibroblast origin that produce abundant extracellular matrix, mainly composed of mucin (mucopolysaccharides) with lesser amounts of collagen. Because of the formation of extracellular matrix material, myxoid tumors may not exfoliate well and cytologic specimens are typically of low cellularity,1 as seen in this case. The streaks of pink to light purple matrix material in the cytologic smears was presumed to be mucin, based on the color and windrowing effect on red blood cells, reminiscent of joint fluid (which is also viscid). Other differential diagnoses for this material potentially include chondroid matrix, glycoproteins, and glycosaminoglycans.1,2 However, in this case, the color and nature of the material was not consistent with chondroid, which is darker red-purple and more granular versus being in linear strands. The presence of individualized spindle cells within mucinous matrix material should increase suspicion of a myxomatous tumor and supports that the cells are of fibroblast origin. However, there are many mesenchymal tumors that are capable of mucin production, including soft tissue sarcomas (e.g. nerve sheath tumors), superficial and deep angiomyxoma, myxoid liposarcoma, low-grade fibromyxoid sarcoma, myxofibrosarcoma, myoepithelial carcinoma/mixed tumor (including mammary forms), extraskeletal myxoid chondrosarcoma, and chordoma.2,3 Myxoma or myxosarcoma were the top 2 differential diagnoses for the cytologic findings in this case. A myxofibroma was not considered due to lack of awareness of this tumor type and the lack of exfoliated collagen fibrils in the smears, however these fibrils are less likely to exfoliate compared to the softer mucinous matrix. The presence of collagen fibrils within the tumor on histologic examination led to the diagnosis of myxofibroma over myxoma. The latter tumors typically consist of low numbers of mesenchymal cells in an abundant mucinous matrix.2 The anisokaryosis and bi- or multinucleation in the cytologic smears was concerning for a myxosarcoma, however the latter tumor is more cellular, has a higher mitotic rate and more atypia2 than that seen histologically in the neoplasm of this report.
Previous reports of myxomatous tumors in goats are rare. Of over 100 tumors in goats, only a single tumor was an omental myxosarcoma.4 The rarity of this tumor type in goats may be due to lack of reporting (e.g. masses not biopsied) or a true low incidence. Historically, connective tissue neoplasms were thought to be less common in goats than tumors of epithelial origin.5 The most commonly reported tumors in goats are squamous cell carcinomas, cutaneous papillomas, and melanomas.6–9 Squamous cell carcinomas are of special importance in lightly pigmented breeds, such as Angora, Boer, and Saanen goats, particularly those living in sunny regions of the world.5,6,10–12 Often the less pigmented perineal regions are affected in these breeds.9,12 Liposarcomas are also a common sarcoma in this species.6
Both myxosarcomas and their benign counterparts can be locally infiltrative with poorly defined margins. When incised, these tumors may exude a clear viscous fluid.2 Because of their infiltrative nature, complete surgical excision may be difficult. However, they are unlikely to metastasize.2 In this case, the mass was excised with complete margins and had not recurred by the time of this report.
References
- Friedrichs, K. R. & Young, K. M. 7 – Diagnostic Cytopathology in Clinical Oncology. In Withrow and MacEwen’s Small Animal Clinical Oncology (Fifth Edition) (eds. Withrow, S. J., Vail, D. M. & Page, R. L.) 111–130 (W.B. Saunders, 2013).
- Hendrick, M. J. Mesenchymal Tumors of the Skin and Soft Tissues. In Tumors in Domestic Animals (ed. Meuten, D. J.) 142–175 (John Wiley & Sons, Inc., 2016).
- Wei, S., Henderson-Jackson, E., Qian, X. & Bui, M. M. Soft Tissue Tumor Immunohistochemistry Update: Illustrative Examples of Diagnostic Pearls to Avoid Pitfalls. Arch. Pathol. Lab. Med. 141, 1072–1091 (2017).
- Löhr, C. V. One Hundred Two Tumors in 100 Goats (1987–2011). Vet. Pathol. 50, 668–675 (2013).
- Zubaidy, A. J. Caprine Neoplasms in Iraq: Case Reports and Review of the Literature. Vet. Pathol. 13, 460–461 (1976).
- Bastianello, S. S. A survey of neoplasia in domestic species over a 40-year period from 1935 to 1974 in the Republic of South Africa. III. Tumours occurring in pigs and goats. Onderstepoort J. Vet. Res. 50, 25–28 (1983).
- Skin. In Goat Medicine 23–60 (John Wiley & Sons, Ltd, 2009). doi:10.1002/9780813818825.ch2
- Roth, L. & Perdrizet, J. Cutaneous histiocytoma in a goat. Cornell Vet. 75, 303–306 (1985).
- Ramadan, R. O. Squamous cell carcinoma of the perineum of the goat. Br. Vet. J. 131, 347–350 (1975).
- Yeruham, I., Nyska, A., Orgad, U. & Waner, T. Perianal Squamous Cell Carcinoma in Goats. J. Vet. Med. Ser. A 40, 432–436 (1993).
- Bildfell, R. J., Valentine, B. A. & Whitney, K. M. Cutaneous Vasoproliferative Lesions in Goats. Vet. Pathol. 39, 273–277 (2002).
- Thomas, A. D. Skin cancer of the Angora goat in South Africa. PhD dissertation (University of South Africa, 1928).
Author: Carol E. Haak (resident) and edited by Tracy Stokol.
Acknowledgments: The author thanks Dr. Juul-Nielsen for submission of the surgically excised mass and permission to use this case.