Interpretation
Left distal femur: Sarcoma
Explanation
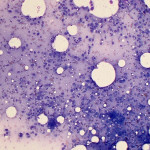
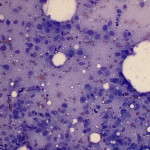
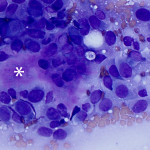
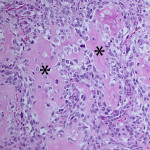
Based on clinical presentation, appendicular osteosarcoma was the leading differential diagnosis (Question 1), but other bone or regional pathology, although less likely, such as a localized histiocytic sarcoma, bone cyst, or granulomatous inflammation are considerations for the clinical presentation (not cytology). The cytologic smear was high cellularity and consists of many individual to aggregates of neoplastic spindle cells (Figure 1). The arrangement and cell shape were compatible with a mesenchymal origin. The neoplastic spindle cells had slightly eccentric, oval nuclei with coarsely stippled chromatin and a moderate amount of cytoplasm with a perinuclear clearing in some cells. These cells displayed multiple cytologic criteria of malignancy, including anisocytosis and anisokaryosis, prominent nucleoli, and mitotic figures, which led to the diagnosis of sarcoma. Further histopathologic examination would be required to definitively specify a type of sarcoma, however, the eosinophilic extracellular matrix was considered compatible with osteoid (Question 3) and osteosarcoma was the top differential diagnosis based on cytologic assessment.
Additional tests
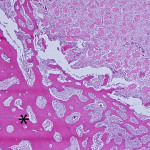
Based on the cytologic results, leg amputation was performed and the diagnosis of osteosarcoma (productive, osteoblastic type) was confirmed on histopathologic examination of the amputated tissue (Figure 4). The marrow space, trabeculae, and cortical bone were infiltrated and replaced by a poorly-demarcated, infiltrative, unencapsulated, densely cellular neoplasm composed of many sheets of neoplastic osteoblasts. The neoplastic osteoblasts were often arranged in short interlacing streams or bundles and surrounded numerous foci of eosinophilic osteoid matrix (Figure 5). The morphologic features of individual neoplastic cells were similar to that seen on the aspirate smears.
Discussion
Canine appendicular osteosarcoma is the most common primary bone neoplasm comprising over 80% of malignant bone tumors.1-3 Approximately 75% of canine osteosarcomas occur in appendicular bone, but they can also occur in the axial skeleton (about 25% of osteosarcomas) or rarely in soft tissue (i.e. mammary gland). The majority of appendicular osteosarcomas arise from endosteum (central osteosarcoma) and are clinically aggressive, with most patients dying or being euthanized due to metastates.1,2
The tumor usually occurs in older dogs, with a mean reported age of 7-8 years in most studies in appendicular osteosarcomas, but it can occur in dogs less than 2 years old.2 Large breed dogs, such as Boxers, Great Danes, Saint Bernards, German Shepherds, and Irish setters are predisposed to developing appendicular osteosarcomas.2 Reported risk factors for tumor development include ionizing radiation, previous trauma, and genetic alterations.1,2 Dogs with osteosarcoma often present with acute or chronic lameness and pain, such as in this case, or bony enlargement.2 High activity of total alkaline phosphatase (ALP) has been recognized as a poor prognostic indicator in some studies of canine and human osteosarcomas.1,2
Osteosarcoma can be frequently diagnosed with the combination of radiographic and cytologic findings.2 Cytologic examination is definitive for a sarcoma in many cases, however, if the tumor is well-differentiated or has unusual features, cytologic assessment may support a tentative diagnosis of sarcoma, which help guide diagnostic and treatment options, such as amputation with histopathologic examination. In one study, the diagnostic accuracy of cytology to distinguish neoplasia from non-neoplasia of bone lesion in dogs was compared to histopathologic results.4 In that study, agreement between cytology and histopathology was seen in 92% of tumor cases and 27% of non-neoplastic cases, with low or poorly cellular samples yielding lower diagnostic concordance for the latter (since tumors are often highly cellular and exfoliate well).4 A definitive diagnosis of osteosarcoma can be supported by performing ALP cytochemical staining of aspirates.5 Osteoblasts generally have high ALP activity (the likely source of high serum ALP activity in some dogs with this tumor) and strong expression of this enzyme is a sensitive and relatively specific marker of this tumor (ALP has also been reported to be positive in an amelanotic melanoma and a chrondrosarcoma, but it is possible the latter tumor was a chondroblastic osteosarcoma).5 Our laboratory does offer this cytochemical stain for the diagnosis of osteosarcoma and acute myeloid leukemia in dogs, however it was not performed on this particular case.
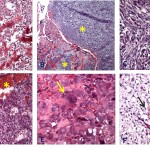
Osteosarcomas are defined as a primary malignant tumor of mesenchymal tissue that can give rise to a variety of histopathologic patterns, but always includes the production of osteoid and/or tumor bone by malignant osteoblasts.3 Histologically, canine osteosarcomas are classified into 7 variants (osteoblastic [productive and non-productive], chondroblastic, fibroblastic, telangiectatic, giant cell, and poorly-differentiated)3 (Figure 6A-F). Histopathologic examination is required for tumor type classification, however, it can be challenging to distinguish chondroblastic osteosarcoma from chondrosarcoma, fibroblastic osteosarcoma from fibrosarcoma and telangiectatic osteosarcoma from hemangiosarcoma when only a small amount of tissue is biopsied or available for evaluation (hint, give pathologists the entire tumor). The histologic subtypes do not appear to affect prognosis in dogs,2,6 but these tumors could have different gene expression which may hold promise for future molecular targeted therapy.
Currently, standard treatment options are leg amputation followed by either a platinum (cisplatin)-or doxorubicin-based chemotherapeutic protocol.1,2 The medium survival time for patients treated with amputation alone is 19 weeks7 and for patients that underwent amputation and cisplatin treatment was 262-413 days.7-9
Canine osteosarcoma is recognized as an animal model for human osteosarcoma and recently, many studies have focused on associated genetic alternations. Investigation of genetic alterations in tumor suppressor genes (i.e. Rb, p53, PTEN)10-13 or overexpression of oncogenes (i.e. erB-2, MET, C-myc), and other growth factors (i.e. IGF-1, VEGF) 14,15 showed several similarities in genetic alterations in humans and canine osteosarcomas. For instance, p53 mutations have been identified in up to 30% of osteosarcoma from human patients and one canine study showed that 47% of 15 canine osteosarcomas also had p53 mutations.15 Ezrin, first described in human and canine osteosarcoma in 2004,16 is a membrane cytoskeleton linker that connects the actin cytoskeleton to extracelluar matrix via transmembrane proteins and has a role in cell to cell interactions and signaling between actin filaments and cell membrane. This protein is associated with pulmonary metastasis, and it is overexpressed in over 80% of tested canine and human osteosarcomas. High ezrin expression is associated with shorter disease free interval and early metastasis in both canine and human osteosarcoma.16 Rapamycin, an mTOR inhibitor and new anti-cancer agent, inhibits ezrin-mediated metastatic behavior in a murine osteoosarcoma model.17 These recently found genetic alterations may become new targets for therapy in future.
References
- Chun, R., et al. 2003. Update on the biology and management of canine osteosarcoma. Vet Clin Sm Anim Prac 33, 491-516.
- Dernell, W. S., et al. 2012 Tumor of the skeletal system. In Small animal clinical oncology. 5th edi. pp 540-582.
- Slayter, M.V., et al. 1994. Malignant tumors. In: Histological classification of bone and joint tumors of domestic animals. World Health Organization, 2nd series, vol 1. Washington (DC): Armed Forces Institute of Pathology.
- Berzina, I., et al. 2008. Correlation between cytologic and histopathologic diagnoses of bone lesions in dogs: a study of the diagnostic accuracy of bone cytology. Vet Clin Pathol 37, 332-338
- Barger, A., et al. 2005. Use of alkaline phosphatase to differentiate canine osteosarcoma from other vimentin-positive tumors. Vet Pathol 42, 161-165.
- Kirpensteijn, J., et al. 2002. Prognostic significance of a new histologic grading system for canine osteosarcoma,Vet Pathol 39, 240-246.
- Mauldin, G.N., e al. 1988. Canine osteosarcoma. Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J Vet Intern Med 2, 177–80.
- Kraegel SA, Madewell BR, Simonson E: Osteogenic sarcoma and cisplatin chemotherapy in dogs: 16 cases (1986–1989), J Am Vet Med Assoc 199:1057-1059, 1991.
- Straw RC, et al. 1991. Amputation and cisplatin for treatment of canine osteosarcoma, J Vet Int Med 5:205-210.
- Sagartz, J.E., et al. 1996. Suppressor protein overexpression in osteogenic tumors of dogs, Vet Pathol 33:213-221.
- Mendoza, S., et al. 1998. Status of the p53, Rb and Mdm2 genes in canine osteosarcoma, Anticancer Res 18:4449-4454.
- Levine, R.A., et al. 2000. Inactivation of p53 and retinoblastoma family pathways in canine osteosarcoma cell lines, Vet Pathol 37:54-61.
- Johnson, A.S. et al. 1998. Mutation of the p53 tumor suppressor gene in spontaneously occurring osteosarcomas of the dog, Carcinogenesis 19,213-217.
- Levine RA, Forest T, Smith C: Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and Tumors, Vet Pathol 39:372-378, 2002.
- MacEwen EG, Pastor J, Kutze J et al: IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma, J Cell Biochem 92:77-91, 2004.
- Khanna, C., et al. 2004. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nature 10, 182-186.
- Wan, X. et al. 2005. Rapamycin inhibits Ezrin-mediated metastatic bahavior in a murine model of osteosarcoma. Cancer Res 65, 2406.
Authored by: M Asakawa (clinical pathology resident) and T Stokol. The authors thank Drs. Heidi Lee Pecoraro and Andrew Miller, the anatomic pathologists who shared the histopathologic findings of this case.