Interpretation
Chronic lymphocytic leukemia
Explanation
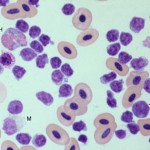
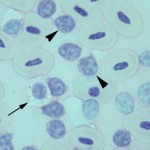
Smear examination revealed many leukocytes (high leukocyte count), consisting mostly of small lymphocytes (96%), with a few large lymphocytes. There were low numbers of heterophils (3%), basophils (1%) and monocytes. No polychromasia was evident, supporting a non-regenerative anemia (Figure 1a). Thrombocytes were considered to be low. The lymphocytes were negative on staining with Periodic acid-Schiff (Figure 2). A leukocyte count was markedly increased in the duck; a total leukocyte count of 600,000/µL was obtained using the Eosinophil Unopette method for leukocyte determination in avian species.1 Based on the extreme leukocytosis of small lymphocytes, a diagnosis of chronic lymphocytic leukemia was made. Direct smears of the joint fluid aspirate were cellular, with an estimated total nucleated cell count of 15,000-20,000/µL and minimal blood. Small lymphocytes were the dominant cell population in the joint fluid smears. Serum total protein electrophoresis demonstrated that the high total protein consisted of a marked polyclonal gammopathy. There was a concurrent hypoalbuminemia. A bone marrow aspirate was highly cellular, with many small lymphocytes and low numbers of differentiating granulocytes and erythroid cells.
Discussion
Chronic lymphocytic leukemia (CLL) is a clonal neoplastic disorder of mature lymphocytes and is most frequently diagnosed in dogs and cats.3 There are isolated reports of chronic lymphocytic leukemia in birds, two of which have been of T cell origin.4-7 Leukemia is defined as the presence of neoplastic hematopoietic cells in blood and/or bone marrow and is further subclassified into acute or chronic leukemia or lymphoid or myeloid leukemia, based on the degree and lineage of differentiation of the neoplastic cells. Chronic leukemias consist of a leukocytosis of well-differentiated cells that can be identified as lymphoid or myeloid based on morphologic features alone. Chronic myeloid leukemias arise in the bone marrow, whereas chronic lymphoid leukemias arise in peripheral lymphoid organs, including the spleen, and home to the bone marrow (particularly with B cell variants).8 Chronic lymphoid leukemia is the most common type of chronic leukemia in animals, with chronic myeloid leukemias being exceedingly rare. Although the term “chronic” applies to the degree of differentiation, these leukemias are usually indolent and slowly progressive (hence the term “chronic” also implies duration and a better prognosis compared to acute leukemias which consist of immature poorly differentiated cells and are of rapid onset and progession and have a poor prognosis). Chronic lymphocytic leukemia is frequently diagnosed in dogs and cats as an inadvertant finding in animals presenting for disorders other than the leukemia.3 Avian viruses, including herpes virus (Marek’s disease) and various retroviruses (leukosis/sarcoma complex) can cause different types of leukemia in birds,9 however an underlying viral infection was not conclusively identified in any of the birds in the published reports of chronic lymphocytic leukemia or in the bird of this report. Hyperglobulinemia was the only biochemical abnormality in the bird of this report and consisted of a polyclonal gammopathy. Hyperglobulinemia has been identified in some but not all of the reported cases of CLL in birds.4,6,7 Dogs with CLL can also have hyperglobulinemia, which is frequently associated with a monoclonal gammopathy of IgG or IgM.3 The cause of the polyclonal gammopathy in this bird was not ascertained. The non-regenerative anemia and suspected thrombocytopenia in this duck was attributed to myelophthisis from the extensive bone marrow infiltrate. The high lymphocyte count in the joint fluid aspirate supported infiltration of the joint capsule by the leukemic cells.
Follow up
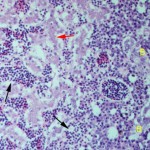
The bird was treated with chlorambucil and prednisolone, both of which have been used to treat other birds with this syndrome, with limited success.4-7 Response to treatment was monitored by measuring a leukocrit, because the Unopette method (which is based on a differential leukocyte count) was considered quite inaccurate with the extremely high leukocyte count. The leukocrit on admission was 33% (Figure 3). Unfortunately, the bird developed an acute onset of vestibular ataxia and died 2 months after diagnosis. A hemogram had been performed 4 days prior to death; the leukocrit had decreased to 20% but the anemia had worsened (the packed cell volume was 13%) and the bird was persistently thrombocytopenic. A necropsy was performed and gross examination revealed a thin bird with hepatomegaly, splenomegaly and several 1 mm whitish nodules in the myocardium. Histologic evaluation revealed extensive sinusoidal and red pulp infiltrates of small lymphocytes in the liver (Figure 4) and spleen, respectively. There were diffuse intravascular and perivascular lymphoid infiltrates in most examined organs. In the right hock, lymphocytes extended perivascularly into synovial tissue, supporting the cytologic finding of synovial infiltrates. The bone marrow was hypercellular with extensive diffuse infiltrates of small lymphocytes. There were low numbers of plasma cells and foci of heterophilic precursors. The white nodules in the myocardium corresponded to a multifocal necrotizing myocarditis with intralesional coccobacilli (this was attributed to a terminal septicemia). At the time of this report, reagents for immunophenotyping lymphoid neoplasms in birds were not available, hence it is unknown if this leukemia was of B or T cell origin.
References
- Campbell TW and Ellis CK. In: Avian and exotic hematology and cytology, 3rd ed. Blackwell Publishing Ltd. 2007.
- Swayne DE, Stockham SL and Johnson GS. Cytochemical properties of chicken blood cells resembling thrombocytes and lymphocytes. 1986; Vet Clin Pathol, 15:17-24.
- Workman HC and Vernau W. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. 2003; Vet Clin North Am Small Anim Pract, 33:1379-1399.
- Hammond EE, et al. Long-term treatment of chronic lymphocytic leukemia in a green-winged macaw (Ara chloroptera). 2010; J Avian Med Surg, 24:330-338.
- Osofsky A, et al. T-cell chronic lymphocytic leukemia in a double yellow-headed Amazon parrot (Amazona ochrocephala oratrix). 2011; J Avian Med Surg, 25:286-294.
- Gaharan S, et al. What is your diagnosis? Chronic lymphocytic leukemia. 2012; J Avian Med Surg, 26:45-48.
- Newell SM, McMillan MC and Moore FM. Diagnosis and treatment of chronic lymphocytic leukemia in a Pekin Duck (Anas platyrhynchos domesticus). 1991; J Assoc Avian Vet, 5:83-86.
- Swerdlow S, et al. WHO classification of haemotopoietic and lymphoid tissue, 4th ed. 2008. WHO.
- Campbell TW. Hematology. In: Avian Medicine, Principles and Application. Ritchie BW, Harrison GW, and Harrison LR, eds. Wingers Publishing. 1994.