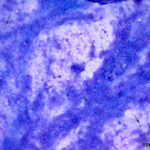
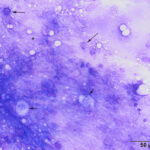
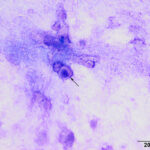
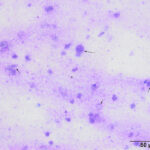
Interpretation
Epithelial necrosis; suspect viral infection
Explanation
The direct smear contained thick stringy mucus, with numerous enmeshed epithelial cells, which were undergoing necrosis and appeared to be fragmenting (Figures 1-4, Question 1). In regions of the smear, there were mildly increased inflammatory cells, consisting of a mixture of non-degenerate neutrophils and macrophages with fewer small lymphocytes (not shown). No infectious agents were identified. The cytologic diagnosis was epithelial necrosis (Question 2) with mild mixed inflammation; likely tracheitis, bronchitis or bronchiolitis (based on the presence of cells with a columnar appearance). The necrosis was suspicious for an underlying viral infection. Considering that several animals had similar respiratory systems and New York City was in the middle of a state-wide shutdown due to the pandemic caused by severe acute respiratory syndrome (SARS)-associated coronavirus-2 (SARS-COV-2), the latter virus was considered to be the likely cause of the infection in the affected tiger.
Additional tests
Thoracic radiographs revealed a bronchial pattern, whereas thoracic ultrasonography showed alveolar-interstitial syndrome1 (seen with various diseases involving the alveoli and interstitium, e.g. pulmonary edema). A PCR-based infectious disease panel for feline pathogens (including Mycoplasma cynos and felis, Bordetella bronchoseptica, pneumovirus, Streptococcus equi subspecies zooepidemicus, and Chlamydophila) was negative. SARS-CoV-2 RNA was amplified from the tracheal wash sample at 2 different laboratories (Animal Health Diagnostic Center at Cornell University, University of Illinois Veterinary Diagnostic Laboratory) and confirmed by the National Veterinary Services Laboratory. SARS-CoV-2 was isolated from the tracheal wash. In situ RNA hybridization using probes for SARS-CoV-2 was performed on the tracheal wash at the University of Illinois. Positive RNA staining was seen in macrophages and epithelial cells, with expected results for negative controls, including cells infected with feline coronavirus (feline infectious peritonitis).2 Taken together, these tests confirmed active SARS-CoV-2 infection in the cat with corresponding lung pathology.
Discussion
SARS-CoV-2 is a single stranded enveloped RNA virus, that is closely phylogenetically related to the original SARS-CoV-1 identified in 2002.3 This strain is thought to have originated from a bat, with the pangolin as a possible intermediate host.4,5 The receptor for SARS-CoV-1 and -2 is the transmembrane protein, angiotensin-converting enzyme 2 (ACE2), which is upregulated by interferon-α and interferon-γ and toll-like receptors.6,7 SARS-CoV-2 has a higher affinity for human ACE2 than SARS-Cov-1, likely explaining its higher infectivity.8 The virus binds to ACE2 via the first region of the envelope spike (S) protein, which undergoes a conformational change once cleaved by cellular proteases, e.g. transmembrane protease serine 2 (TMPRSS2) or cathepsin B or L, facilitating cell fusion and entry.8–10 Amino acid sequence homology to human ACE2 and ACE2 tissue expression largely dictates susceptibility of animals to infection, with ferrets, cats, minks, hamsters and macaques serving as useful experimental models.11–18 Humans can also be a source of infection for these species1,2,19,20 and animal-to-human transmission has been documented for farmed mink.21 In contrast, dogs, chickens, ducks, pigs, cattle (calves) and marmosets are less susceptible to the virus,11,22 although replicate studies with higher viral doses and different viral genotypes are needed. As yet, no infectivity studies have been reported for horses and camelids.
The virus is shed primarily through direct contact and aerosolized droplets of nasal and oropharyngeal secretions, which contain high concentrations of infectious virus and viral RNA.2,11 Feces can also contain viral RNA2,11,12 as well as infectious virus.2 Pathologic findings have largely been described for severely ill human patients that died with symptoms of COVID-19 and likely represent the most severe disease manifestation. Abnormalities are concentrated in the lungs and consist of tracheobronchitis and diffuse alveolar disease (DAD), with microvascular thrombi.23–25 DAD has acute and organizing phases, characterized by edema, hyaline membrane formation and inflammation, and fibrosis and type II pneumocyte hyperplasia, respectively. Both phases may be present in individual patients.24,25 Viral antigen can be found in upper and lower airway respiratory and submucosal glandular epithelium, type I and type II pneumocytes, hyaline membranes and macrophages in the lungs and regional lymph nodes.24 Similarly, viral RNA was detected in macrophages and epithelial cells by in situ hybridization in the tracheal wash of the tiger in this report.2 The inflammatory infiltrate in the lungs of affected human patients consist of macrophages and lymphocytes, which are a mixture of CD4 and CD8 T cells. Neutrophils are thought to reflect secondary bacterial infection.24,25 We saw a similar, albeit mild, inflammatory infiltrate in the tracheal wash of this tiger. The lung pathology in humans is most closely recapitulated in the golden Syrian hamster after high dose infection (106 plaque forming unit/mL).13 Juvenile cats between 6 weeks and 4 months of age can also have substantial pulmonary pathology after high dose infection, with some features of DAD, despite mild clinical signs.11
To our knowledge, this is the first description of cytologic findings in a tracheal wash sample from a patient (animal or human) with SARS-CoV-2 infection, with epithelial necrosis being the predominant finding. The sheer number of dying cells and/or fragments thereof was quite remarkable. Based on distinct columnar shapes, the epithelial cells were thought to have originated from the trachea, bronchi or bronchiole. The clinical signs demonstrated by the tiger were also suspected to be tracheal in origin.1 However, an alveolar origin for the cells is also possible, because there were a few cuboidal variants (the latter could also be fragments of larger cells) and the animal lacked radiographic signs of pneumonia.1 ACE2, the main receptor for SARS, is found in all surface pulmonary epithelia in domestic cats, which conceivably are similarly susceptible to infection if cellular proteases are present to cleave and activate the spike protein. Tracheal (surface epithelium and submucosal gland) epithelial necrosis has been reported in experimentally infected cats.11 Necrosis and mixed inflammation with neutrophils and macrophages was identified in alveolar tissue after experimental infection with SARS-CoV-1 infection of cats.26 Bronchiolar epithelial necrosis has also been described in experimentally infected ferrets with SARS-CoV-2.18 These data corroborate our cytologic findings of necrosis in the tracheal wash from the tiger in this report. It is important to distinguish necrosis from cell degradation with storage. Even though the provided tracheal wash fluid from the tiger was 24 hours old at the time of smear preparation, the cells lacked storage-associated changes, such as nuclear swelling, and rather contained fading nuclei, a feature more compatible with cell death. Detection of viral RNA with in situ hybridization in the epithelial cells2 also supported viral-induced necrosis. In our experience, epithelial necrosis is an uncommon finding in tracheal wash fluid, with a viral infection being the top differential diagnosis. Epithelial necrosis has been reported with SARS-CoV-1, influenza, orthopox and feline herpes virus type-1 infections in cats.26–29 Necrosis and inflammation, however, are not specific for viral infections and can occur with protozoal, fungal and bacterial infections, inhalant toxic injury, ischemic necrosis, and primary or metastatic pulmonary tumors.30–33 There are reports of bronchoalveolar fluid cytologic findings in human patients, which demonstrate an alveolitis based on neutrophilic inflammation, however epithelial necrosis was not described.34,35
Viral RNA was amplified from the tracheal wash of the tiger and fecal samples from the other affected tigers and lions, confirming infection with SARS-CoV-2. Infectious virus was also retrieved from the tracheal wash sample of the tiger and a fecal sample from a lion and another tiger. Prolonged fecal shedding of viral RNA was observed. The animals were believed to have been infected by an asymptomatic handler, who was infected with the virus. Appropriate safety precautions were employed during collection and handling of the samples from all animals.1-2 This case illustrates how a tracheal wash sample provided a unique window into the airways of the tiger and helped facilitate the diagnosis of SARS-CoV-2.
References
- Bartlett SL, Diel DG, Wang L, et al. SARS-COV-2 infection and longitudinal fecal screening in Malayan Tigers (Panthera tigris jacksoni), Amur Tigers (Panthera tigris altaica), and African Lions (Panthera leo krugeri) at the Bronx Zoo, New York, USA. J Zoo Wildl Med 2021;51:733–744.
- McAloose D, Laverack M, Wang L, et al. From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio 2020;11:e02220-20. doi: 10.1128/mBio.02220-20..
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536–544.
- Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nature Medicine 2020;26:450–452.
- Xiao K, Zhai J, Feng Y, et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature 2020;583:286–289.
- Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020;181:1016-1035.e19.
- Aboudounya MM, Heads RJ. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediators Inflamm 2021;2021:8874339.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020;181:271-280.e8.
- Pišlar A, Mitrović A, Sabotič J, et al. The role of cysteine peptidases in coronavirus cell entry and replication: The therapeutic potential of cathepsin inhibitors. PLoS Pathog 2020;16:e1009013.
- Padmanabhan P, Desikan R, Dixit NM. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. PLoS Comput Biol 2020;16:e1008461.
- Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020;368:1016–1020.
- Kim Y-I, Kim S-G, Kim S-M, et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020;27:704–709.
- Chan JF-W, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 2020;71:2428-2446.
- Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020;368:1012–1015.
- Richard M, Kok A, de Meulder D, et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nature Communications 2020;11:3496.
- Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020;369:812-817.
- Welkers MRA, Han AX, Reusken CBEM, et al. Possible host-adaptation of SARS-CoV-2 due to improved ACE2 receptor binding in mink. Virus Evol 2021;7:veaa094.
- Ryan KA, Bewley KR, Fotheringham SA, et al. Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity. Nat Commun 2021;12:81.
- Neira V, Brito B, Agüero B, et al. A household case evidences shorter shedding of SARS-CoV-2 in naturally infected cats compared to their human owners. Emerg Microbes Infect 2020:1–22.
- Hamer SA, Pauvolid-Corrêa A, Zecca IB, et al. Natural SARS-CoV-2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID-19 cases in Texas, USA. bioRxiv 2020. Dec 8;2020.12.08.416339. doi: 10.1101/2020.12.08.416339. Preprint.
- Hammer AS, Quaade ML, Rasmussen TB, et al. SARS-CoV-2 Transmission between Mink (Neovison vison) and Humans, Denmark. Emerg Infect Dis 2021;27:547–551.
- Ulrich L, Wernicke K, Hoffman D, et al. Experimental infection of cattle with SARS-CoV-2. Emerg Infect Dis 2020;26:2979-2981. https://dx.doi.org/10.3201/eid2612.203799
- Sadegh Beigee F, Pourabdollah Toutkaboni M, Khalili N, et al. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol Res Pract 2020;216:153228.
- Martines RB, Ritter JM, Matkovic E, et al. Early Release – Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States – Volume 26, Number 9—September 2020 – Emerging Infectious Diseases journal – CDC. Available at: https://wwwnc.cdc.gov/eid/article/26/9/20-2095_article. Accessed May 21, 2020.
- Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020;8:681–686.
- van den Brand JMA, Haagmans BL, Leijten L, et al. Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet Pathol 2008;45:551–562.
- Hinrichs U, van de Poel H, van den Ingh TS. Necrotizing pneumonia in a cat caused by an orthopox virus. J Comp Pathol 1999;121:191–196.
- McGregor L, Martin J, McGregor JL. Pneumonitis and gastritis in a cat with feline herpesvirus-1. Can Vet J 2016;57:147–150.
- Löhr CV, DeBess EE, Baker RJ, et al. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Vet Pathol 2010;47:378–386.
- Sura R, Van Kruiningen HJ, DebRoy C, et al. Extraintestinal pathogenic Escherichia coli-induced acute necrotizing pneumonia in cats. Zoonoses Public Health 2007;54:307–313.
- Carvallo FR, Debroy C, Baeza E, et al. Necrotizing pneumonia and pleuritis associated with extraintestinal pathogenic Escherichia coli in a tiger (Panthera tigris) cub. J Vet Diagn Invest 2010;22:136–140.
- Fox JG, Murphy JC, Shalev M. Systemic fungal infections in cats. J Am Vet Med Assoc 1978;173:1191–1195.
- Parker GA, Langloss JM, Dubey JP, et al. Pathogenesis of acute toxoplasmosis in specific-pathogen-free cats. Vet Pathol 1981;18:786–803.
- Pandolfi L, Fossali T, Frangipane V, et al. Broncho-alveolar inflammation in COVID-19 patients: a correlation with clinical outcome. BMC Pulm Med 2020;20:301.
- Vedder V, Schildgen V, Lüsebrink J, et al. Differential cytology profiles in bronchoalveolar lavage (BAL) in COVID-19 patients: A descriptive observation and comparison with other corona viruses, Influenza virus, Haemophilus influenzae, and Pneumocystis jirovecii. Medicine (Baltimore) 2021;100:e24256.
Author: Tracy Stokol with many other (D McAloose, S Bartlett, K Terio for complete list, see references #1 and #2)