Interpretation
Mixed inflammation with neutrophils and macrophages due to a bacterial infection.
Explanation
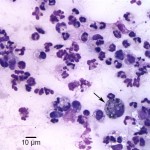
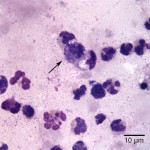
Aspirate smears were moderately to highly cellular and composed of a mixed inflammatory population characterized by numerous neutrophils, some of which were degenerate, as well as fewer but still abundant vacuolated macrophages, within a stippled background with small amounts of blood. Rare macrophages with numerous black-green cytoplasmic granules (melanin) were also seen (Figure 1). Moderate numbers of small coccobacili were found extracellularly and phagocytized within neutrophils (Figure 2 on page 1 and Figure 3 below). The bacteria were gram negative on a gram stain (Figure 4). A cytologic diagnosis of mixed inflammation with bacterial infection was made (Question 1).
The morphologic features of the bacteria are compatible with both Mycoplasma and Brucella species, which are characterized by their small size (0.3-0.8 μm in diameter) compared to other conventional bacteria. Mycoplasmas have a more variable appearance, ranging from coccoid to coccobacilli to signet ring, whereas Brucella species are less pleomorphic and are mostly coccobacilli (Question 2). Further testing (besides general bacterial and mycoplasmal cultures) includes serological tests, such as Rapid Slide Agglutination Test (RSAT) and Tube Agglutination Test (TAT) for Brucella (Question 3).
Additional information
Fungal and mycoplasmal cultures of the ocular samples were negative. Polymerase chain reaction testing was performed on aqueous humor and blood samples and amplified Brucella canis DNA. Serological tests for brucellosis; RSAT, agarose gel immunodiffusion (AGID), TAT and indirect immunofluorescent antibody (IFA) were all positive (both latter assays at 1:200; high titer). A final diagnosis of uveitis (endophthalmitis) due to chronic infection with Brucella canis was made.
Discussion
In dogs, brucellosis is mainly caused by Brucella canis, a Gram-negative, aerobic, nonmotile, nonspore-forming, and facultatively intracellular coccobacillus. Occasional infections with Brucella melitensis, Brucella suis, and Brucella abortus have also been described.1,2 B. canis routes of entry are through the genital, oronasal or conjuctival mucosa, but entry through broken skin may also occur.1,2 The highest concentrations of bacteria are found in vaginal secretions, semen and aborted material from infected dogs. The bacteria persist in vaginal discharges and semen for weeks or months after infection.1-4 Lower concentrations of bacteria have been isolated from saliva, nasal and ocular secretions, blood, milk, and feces.1,2 B. canis can also be shed in urine, with higher concentrations isolated from the urine of male dogs.1 Puppies can be infected in utero or from milk during nursing.2 Another possible source of infection are fomites. Brucella spp. remains viable in the environment for several months, manifesting a longer survival when temperature is low.2
Once Brucella gains entry into the host, the bacteria are phagocytized by macrophages and transported to lymphatic and genital tissues, where they multiply, and a leukocyte-associated bacteremia develops within two to three weeks after infection.1-4 The bacteremia persists for at least 6 months and then occurs intermittently for up to 64 months in chronically infected dogs. After reaching target organs, bacteria produce the pathological changes associated with the disease, which result from localization of tissues and immune-mediated injury.1,3 In intact dogs, canine brucellosis is mostly associated with reproductive conditions such as abortion, infertility, testicular atrophy, prostatitis and epididymitis. Infection in prepubescent males and females mainly manifests as generalized lymphadenopathy, along with splenomegaly and hepatomegaly.4 However, some infected dogs are asymptomatic or have nonspecific signs such as poor hair coat, lethargy, weight loss, behavioral changes, and exercise intolerance.
Other clinical signs associated with B. canis infection are dermatitis, osteomyelitis, endocarditis, meningoencephalitis, and visceral abscesses. Fever is rarely present.1,2 Focal signs such as diskopondylitis and chronic ocular signs in the absence of clinical disease have also been reported in spayed or neutered animals.2-6 Clinical ocular lesions reported to occur in dogs that have been naturally and experimentally infected with B. canis include anterior uveitis, chorioretinitis, paneuveitis, retinal detachment, endophthalmitis, panophthalmitis, vitritis, and keratoconjuctivitis.3,5,7 Ocular inflammation is most frequently unilateral and often associated with intraocular hemorrhage due to breakdown of the blood aqueous barrier and the severe inflammation.3,7 The reason for the unilateral presentation is not completely clear; differences in anatomic or physiologic integrity of the blood aqueous barrier between eyes of dogs with brucellosis as well as bilateral subclinical ocular disease have been proposed as plausible explanations.3 The prognosis for canine ocular brucellosis is generally regarded as poor, presumably as a consequence of sequestration of bacteria within the globe and the relative inaccessibility of the eye to antimicrobials.3,8
Diagnostic tests to determine the cause of uveitis should include a complete ophthalmic and physical examination, complete blood count, serum biochemistry panel, urinalysis, imaging studies of the thorax and abdomen, ocular ultrasound, infectious disease serology, microbiology if indicated, histopathology, and aqueous and/or vitreous aspiration cytology.9 Cytologically Brucella canis is characterized by its small size (0.6-1.5 μm x 0.5-0.7 μm) compared to other bacteria. However, Mycoplasma microorganisms, which are considered as part of the normal microbial flora of the canine conjunctiva, share this same characteristic size; although mycoplasmas have a more variable appearance, as mentioned above. Mycoplasma microorganisms can also be seen as small basophilic granules adherent to surface membrane of epithelial cells or they can extend off epithelial cells into the background. They are rarely seen phagocytized within inflammatory cells, which contrasts with the numerous phagocytized bacteria in this case.10-12 Brucella spp. induces a chronic inflammatory response, consisting of both neutrophils and macrophages, as seen in the present case. Bacteria can be seen within the cytoplasm of the inflammatory cells or free in the background.
A definitive diagnosis of Brucellosis is only achieved through bacterial isolation or molecular detection of the microorganism. These methods can be performed antemortem in blood, urine, semen vaginal discharge, gonadal tissue, intervertebral disk, bone, or ocular fluids.1,3 Brucella spp. can be isolated on various plain or selective media such as Farrell’s medium or Thayer-Martin’s modified medium. Brucella canis is differentiated from other species of the genus Brucella (except Brucella ovis) due to its characteristic rough and mucoid colony morphology. Growth is slow, which makes detection more difficult. Blood cultures are likely to be the least contaminated with other organisms. Temporary absence of bacteria in the tissue cultured, the intermittent nature of the bacteremia, loss of bacterial viability during sample handling, and overgrowth of contaminant bacteria can result in a false negative result. Therefore, repeated culture may be necessary for successful isolation.1-3
Serum antibodies against Brucella are usually detectable at 2 weeks post-infection and develop against the wall (e.g. lipopolysaccharide) and cytoplasmic proteins of the bacteria.1 Serological tests for the detection of antibodies against Brucella canis include RSAT and TAT. Differences in sensitivity and specificity between assays at different stages of infection make interpretation problematic.1,2,5 Both RSAT and TAT can be performed with or without the addition of 2-mercaptoethanol, which improves specificity by dissociating IgM (less specific antibody). However, this can also decrease sensitivity during early stages of the immune response.1 Positive reactions in some screening tests, such as RSAT, are often confirmed with a more specific assay. Cross-reactions with other Gram-negative bacteria (Bordetella and Pseudomonas) can occur with RSAT and nonspecific agglutination reactions have been reported. False negative reactions can occur in early stages of infection as well as in chronically infected animals; situations in which antibodies against B. canis may be absent.2 Other serological tests used mainly in research settings or in individual clinical cases include AGID, IFA, ELISA, Lateral Flow Immune-chromatographic assay (LFIA), complement fixation, and counter-immunoelectrophoresis.1,2
According to a previous study, the most common infectious organisms associated with uveitis in the dogs are Ehrlichia canis (38% prevalence) and Blastomycosis dermatitidis (27% prevalence).9 However, it should be noted that despite the few numbers of published reports of canine ocular brucellosis, Brucella canis has been established and recognized as a cause of intraocular inflammation. Furthermore, a report showed that of 313 dogs with brucellosis, 38 had ocular lesions.7 Therefore, when dogs are presented with clinical signs of uveitis, Brucella canis should be considered among the possible underlying infectious cause of the inflammation.
Case Follow-up
Additional antimicrobial treatment with enrofloxacin, doxycycline, and streptomycin was initiated and continued until all serologic assays were negative (after 112 weeks of systemic treatment). Six months following achievement of seronegativity, there was no recurrence of ocular lesions; likewise both serological assays and blood cultures remained negative.
References
- Wanke M. Canine brucellosis. 2004. Anim Reprod Sci. 82-83. 195-207
- Spickler A, Canine Brucellosis: Brucella canis. 2012. At http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php
- Ledbetter E. et al. Brucella canis endophthalmitis in 3 dogs: clinical features, diagnosis, and treatment. 2009. Vet Ophthalmol. 12. 183-191
- Megid J. et al. Clinical Manifestations of Brucellosis in Domestic Animals and Humans. 2010. Open Vet. J. 4. 119-126
- Mosallanejad B. et al. Brucella canis-induced anterior uveitis in companion dogs in Ahvaz, Iran. 2013. Comp Clin Pathol. At www.springer.com
- Junzo S. et al. Ocular Lesions in Experimental Canine Brucellosis. 1977. Jap. J. vet. Sci. 39. 181-185
- Vinayak A. et al. Clinical resolution of Brucella canis-induced ocular inflammation in a dog. 2004. J Am. Med. Assoc. 224. 1804-1807.
- Green C. et al. Chapter 38. In: Green C, ed. Infectious Diseases of the dog and cat. 4th ed. St. Louis, MO: Saunders Elsevier, 2012
- Massa KL et al. Causes of uveitis in dogs: 102 cases (1989-2000). 2002. Vet Ophthalmol. 5. 93-98
- Ülgen M. et al. Urinary Tract Infections due to Mycoplasma canis in Dogs. 2006. J Vet Med A Physiol Pathol Clin Med. 53. 379-382
- English K. et al. Transtracheal and Bronchoalveolar washes, Chapter 16. In: Cowell R and Valenciano A, ed. Diagnostic Cytology and Hematology of the Dog and Cat. 3rd ed. St. Louis, MO: Saunders Elsevier, 2006
- Raskin R. Eyes and Adnexa, Chapter 15. In: Raskin R and Meyer DJ, ed. Canine and Feline Cytology: a Color Atlas and Interpretation Guide. 2nd ed. St. Louis, MO: Saunders, Elsevier, 2010
Authors: D Hernandez, T Stokol
This case was published as part of a case series: Ledbetter E et al 2009 Vet Ophthalmol. 12:183-191.