Interpretation
Nematode lungworm infection
Marked mixed inflammation (eosinophils, neutrophils)
Explanation
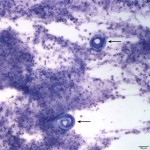
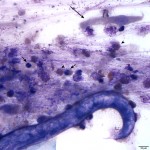
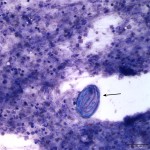
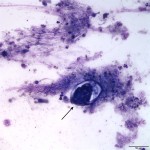
The sediment smears were highly cellular consisting of numerous mixed inflammatory cells and moderate numbers of respiratory epithelial cells on a background of a moderate amount of mucus and few red blood cells. The inflammatory cells were mostly neutrophils (59%) and eosinophils (40%) with rare macrophages (1%) (Question 1). The swollen appearance of the neutrophils was interpreted as an aging artifact due to sample storage, rather than degenerate change. At low power (10x objective), several lungworm larvae and eggs could be seen (Question 2). The only lungworm that infects cattle is Dictyocaulus viviparus (Question 2). Several larvated eggs (Figure 3) and a single freshly laid egg (Figure 4) were noted. In addition, there were moderate numbers of extracellular mixed bacteria (not shown). No intracellular bacteria were identified to confirm a concurrent bacterial infection.
Documentation of the larvae (as well as eggs) in a tracheal wash sample is consistent with a patent infection. A patent infection indicates the presence of mature adult lungworms capable of laying eggs in the host. The other diagnostic test used to diagnose a patent infection is the Baermann technique on fresh feces obtained on rectal exam (Question 3).1 It is not recommended to obtain feces from the ground, as environmental contamination can confound results. The Baermann test identifies first-stage larvae, which are approximately 300 – 360 μm long.2 Alternatively, identification of the white long (up to 8.0 cm in length) mature adult lungworms in the bronchi, mainly in the caudal lobes, on necropsy is also confirmation of a patent infection.1,3,4
Case Follow-up
The aerobic bacterial culture grew moderate numbers of Mannheimia haemolytica, which was susceptible to nearly all antibiotics. The viral panel testing for bovine respiratory syncytial virus, infectious bovine rhinotracheitis, bovine coronavirus, and bovine viral diarrhea virus yielded all negative results. Per communication with the submitting veterinarian, the herd was treated with eprinomectin for the lungworm infection and continued antibiotics for the secondary bacterial infection. Most animals have recovered, however about six animals continue to have mild clinical signs.
Discussion
Dictyocaulus viviparus, the lungworm of cattle, causes a parasitic / verminous pneumonia and bronchiolitis in young, nonimmune calves and in previously unexposed yearling or adult cattle, primarily in the Southern United States.1,3,5,6 The genus Dictyocaulus is present within the superfamily Trichostrongyloidea, the order Strongylida, and phylum Nematoda.3 Other Dictyocaulus species include D. filaria (sheep, goat, camel), D. cameli (camel), and D. arnfieldi (donkeys, horses).2 D. viviparus can also infect camelids.2 All other lungworm species belong to the superfamily Metastrongyloidea.3
Dictyocaulus viviparus has a direct life cycle. Female adults lay eggs, which contain a first-stage larva that typically hatch prior to being passed in the feces of the host.1,3 Cattle cough up the egg and/or first-stage larva, swallow them, and pass them in the feces.6 Development from the first-stage to infective third-stage larvae takes about 5 days in optimal moist conditions.3,5 The larvae are thought to survive on stored food materials, rather than ingested bacteria from the environment, supported by their development in aerated clean water and the gradual disappearance of their “food granules” in the intestinal cells with development.3 The host is infected by ingesting third-stage larvae from the contaminated environment.1,3 Ingested larvae cross the intestinal wall to the mesenteric lymph nodes, where they moult to the fourth stage.1 Within 5-7 days, the fourth-stage larvae migrate to the lungs via the lymphatics and thoracic duct. The larvae then mature to the final fifth-stage mature egg-laying adults in the bronchioles.1,6 The prepatent period (time from ingesting the infective third-stage larvae to development of egg-laying adults and the presence of first-stage larvae in feces) is approximately 4 weeks, and corresponds to the peak of clinical signs.1,3
Light infections with D. viviparus result in occasional coughing with possible mild tachypnea.3 Heavier infestations lead to a progressive increase in respiratory rate, severe coughing, a characteristic deep and moist cough, and moist rales or crackles heard over the entire lung field, as were present in this case.1,3 The coughing is noted to be more severe than with most other causes of bovine pneumonias.1 The auscultation of diffuse rales, rather than rales limited to the anterior ventral lung fields is another important differentiating factor between parasitic and bacterial pneumonia.1 Affected cows may also demonstrate increased expiratory and inspiratory effort. Fever is inconsistently present, and may be due to a secondary opportunistic bacterial infection, most commonly caused by Pasteurella multocida, or due to respiratory exertion in warm weather or poorly ventilated conditions.1 Tracheal wash cytologic findings also help to differentiate parasitic versus primary bacterial pneumonia. Eosinophilic inflammation strongly suggests a parasitic infection, whereas suppurative inflammation is more supportive of a bacterial infection. The nearly equal proportions of neutrophils and eosinophils in this case, may be due to the concurrent lungworm infection and secondary bacterial infection. A tracheal wash cytology with eosinophilic inflammation and no larvae could indicate a postpatent infection.1
Pulmonary lesions in affected cattle range from interstitial pneumonia caused by migrating larvae in the prepatent period to eosinophilic and neutrophilic bronchitis in the patent period with the presence of intrabronchial adult parasites.4,5,7 Eosinophilic granulomas may also occur due to dead larvae, aberrant parasites, or eggs of parasites.4 Adult worms in bronchi cause a substantial loss of ciliated epithelial cells resulting in severe dysfunction of the mucociliary clearance and thus increased susceptibility to infectious and noninfectious pathogens.7 In addition, the flat alveolar epithelium is partially destroyed, and is replaced by hyperplastic cuboidal alveolar type II cells (type II pneumocytes) leading to inhibition of proper gas exchange between the blood and air spaces.7 Heavily affected bronchioles contain abundant amounts of cell-rich mucus, larvae, and eggs which result in atelectasis or consolidation of alveoli.4,7 Interstitial emphysema may also occur due to the forced expirations against a partially obstructed single bronchus.4 At any point during the prepatent, patent, or postpatent period, these life-threatening complications can occur and result in death.6
Treatment consists of anthelmintic therapy to destroy the parasite, and when necessary, as in this case, antibiotics to control the secondary bacterial infection.1 The presence of a secondary bacterial pneumonia gives the clinical impression of partial improvement on antibiotics with resolution of fever and improved attitude, but persistent coughing and dyspnea.1 Infective larvae can survive over the winter; therefore pastures should not be grazed in the early spring.1 Given that moisture promotes the survival and activity of infective larvae, clinical lungworm infections in the northeastern United States typically occur during wet summers.1 This farm reportedly did not deworm this summer.
Veterinarians should also be aware of the reinfection syndrome, which occurs when previously sensitized adult cattle are placed on pastures heavily contaminated with Dictyocaulus.1,4,6 Although most ingested larvae are killed or fail to mature in previously infected cattle, heavy re-exposure allows some larvae to reach the lungs.1,6 Clinical respiratory signs develop 14-16 days after exposure, and are thought to be due to an immune-mediated response to the migrating larvae.1,6 Baermann’s fecal examination is usually negative for Dictyocaulus, because the disease is a result of L4 migration into the lung.1 Tracheal wash samples will reveal eosinophilic inflammation, with possibly fewer lymphocytes, plasma cells, macrophages, and multinucleated giant cells.1 All animals should be treated routinely with anthelmintics if the animals are to be placed on contaminated pastures.1
References
1. Divers TJ. Chapter 4. In: Divers TJ and Peek SF, eds. Rebhun’s Diseases of Dairy Cattle. 2nd ed. St. Louis, MO: Saunders Elsevier, 2008.
2. Zajac AM, Conboy GA. Veterinary Clinical Parasitology. 8th ed. Ames, IA: Wiley Blackwell, 2012.
3. Lopez A. Chapter 9. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Saunders Elsevier, 2012.
.4. Bowman DD. Georgis’ Parasitology for Veterinarians. 10th ed. St. Louis, MO: Saunders Elsevier, 2014.
5. Panciera RJ, Confer AW. Pathogenesis and Pathology of Bovine Pneumonia. 2010. Veterinary Clinics of North America: Food Animal Practice. 26 (2). 191-214.
6. Zajac AM. Parasitic Bronchitis and Pneumonia. In: Smith BP, ed. Large Animal Internal Medicine. 4th ed. St. Louis, MO: Mosby Elsevier, 2009.
7. Schnieder T, Kaup FJ, Drommer W. Morphological investigations on the pathology of Dictyocaulus viviparus infections in cattle. 1991. Parasitol Res. 77. 260-265.
Authors: A Newman (Clinical pathology resident), T Stokol