Interpretation
Tumor, suspect hepatoblastoma, with concurrent cholestasis and necrosis.
Explanation
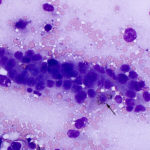
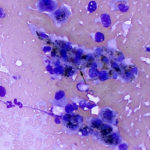
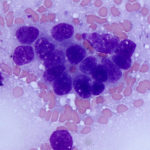
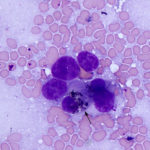
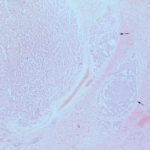
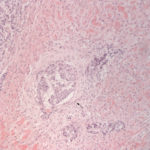
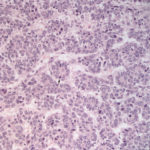
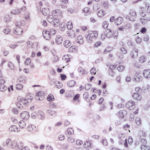
The smears of the liver mass were highly cellular and comprised of dense clusters and cords (Figures 1-2), with acinar-like arrangements (Figures 4, 4a) of medium cuboidal neoplastic epithelial cells with paracentral nuclei and small amounts of medium blue cytoplasm. The cells displayed mild to moderate anisokaryosis and anisocytosis. Most of the tumor cells did not resemble hepatocytes (Question 1), which are larger more polygonal cells with pink granular cytoplasm, found as flat sheets (Figure 3). However rare tumor cells contained light pink cytoplasmic granules and green to green-brown pigment (Figure 5), similar to that seen in the interspersed individualized and small clusters of well-differentiated hepatocytes (Figure 2a), suggesting that they were variants of the theme. Mature hepatocytes contained green to green-brown pigment within their cytoplasm (lipofuscin or bile, presumptive) and bile casts were noted in some clusters (Figure 3, 3a). There was also evidence of necrosis (not shown). Since the tumor cells appeared to be differentiating into hepatocytes, a presumptive cytologic diagnosis of hepatoblastoma was made (Question 2).
Histopathologic examination of a hemotoxylin-&-eosin-stained section of the biopsy revealed nests and rosettes of epithelial cells, supported by a fine fibrovascular stroma. The cells had a high mitotic rate (58 mitoses per 10 high power fields) and there was concurrent necrosis, and mixed, mostly neutrophilic, inflammation in regions of necrosis. A presumptive diagnosis of hepatic carcinoid was made, with a differential diagnosis of a malignant phaeochromocytoma. However, the tumor cells were negative for cytokeratin, neurone-specific enolase (NSE) and synaptophysin, which yielded an alternative tentative diagnosis of hepatoblastoma.
Additional Diagnostic Testing
Based on the cytologic and histopathologic findings of the liver biopsy and persistent erythrocytosis despite aggressive fluid therapy, the owners of the filly were given a poor prognosis and the horse was humanely euthanized. A post-mortem examination revealed a markedly enlarged liver that occupied approximately 30% of the abdominal cavity and comprised approximately 10% of body weight (normal <1%). A large mass (30 x 17 x 25 cm) effaced the left lobe of the liver and was adherent to the omentum (the cranial abdominal mass noted on imaging). The mass had a solid rim with multiple fluid-filled cavities in the center (corresponding to the liquid center noted on ultrasonographic examination). There were very many (>100) other smaller masses scattered throughout the liver lobes. The duodenal and colonic mucosa and enlarged left kidney were diffusely red (congestion) with hemorrhagic luminal contents in the intestine.
Histopathologic examination of hematoxylin-&-eosin-stained sections of formalin-fixed tissue revealed multifocal infiltrative multinodular masses in the hepatic parenchyma that effaced normal architecture. The masses consisted of lobules and trabecular clusters of medium polygonal cells with parancentral nuclei and a moderate amount of pale eosinophilic cytoplasm. The mitotic rate was very high (around 82 in 10 high power fields). Nests of tumor cells were observed within adjacent more normal hepatic parenchyma and within central veins (Figures 6-9). Cystic regions, containing small amounts of proteinaceous fluid, were observed in some masses. There was concurrent bile duct hyperplasia (explaining the increases in GGT activity on the biochemical profile), with plugs of bile (cholestasis). Other sections of the mass contained diffuse regions of coagulative necrosis or large thrombi that occluded the lumen of some veins (not shown). Diffuse congestion was observed in centrilobular regions of the normal liver and other organs (lungs, kidney, sternal lymph node, duodenum and colon). The histologic diagnosis was a hepatic tumor with the two differential diagnoses being a hepatoblastoma and hepatic carcinoid.
Additional sections were immunostained for S-100, NSE, chromagranin A, synaptophysin, pancytokeratin and alpha-fetoprotein. The tumor was diffusely negative for neuroendocrine markers and pancytokeratin, but 10% of the cells were positive for alpha-fetoprotein, confirming the cytologic diagnosis of hepatoblastoma. Frozen serum was subsequently submitted for analysis of erythropoietin concentrations. The erythropoietin concentration was increased at 28 mU/mL (reference interval 1-11.8 mU/mL), supporting a secondary inappropriate erythrocytosis, likely from paraneoplastic production of excess erythropoietin by the tumor (Question 3).
Discussion
Hepatoblastoma is an exceedingly rare tumor of adult human beings,1 being most commonly seen in children under 3 years of age.2 It is the most common tumor in the liver of children and there appears to be a higher risk in premature infants or those of low birthweight.2,3 Even in children, hepatoblastoma is quite uncommon, with a reported incidence rate of 0.25-1.5 per one million (including adults and children).3,4 In animals, hepatoblastoma is most frequently reported in young horses,5-7 including this case, which was a previously published case report,8 with rare reports in other species, including cats and camelids.9,10
The ontogeny of hepatoblastoma is not completely known, but it is thought to arise from primitive hepatoblasts, hepatic stem cells or multipotent hepatic precursors due to mutations in pathways that promote oncogenesis. The various histologic subtypes of hepatoblastoma in people resemble morphologic features in hepatocytes at various stages of differentiation in murine embryos, supporting this theory.3 Recent whole exome sequencing studies have found various mutations within Wnt- and β-catenin-signaling pathway genes including CTNNB1 (β-catenin), adenomatous polyposis coli gene (APC, negatively regulates β-catenin) and AXIN1 (interacts with APC and negatively regulates Wnt signaling) or genes involved in proteasomal-mediated degradation.11,12 These mutations may lead to over-expression of β-catenin, particularly within the nuclei of cancer cells. β-catenin is an important transcriptional regulator of cell proliferation and adhesion and over-expression or activation has been associated with many different cancer types, including hepatoblastoma. Over-expression of β-catenin may occur directly as a consequence of gain-of-function mutations or downregulation of degradation via the proteasome. In addition, abnormalities in the gene NFE2L2 and its signaling protein product, Nrf2, which is involved in antioxidant functions, have been seen in hepatoblastoma and may be mediated by aberrant signaling induced by β-catenin.13 However, other aberrant molecular pathways may also be involved, including loss of tumor suppressor genes.14
The main presenting clinical signs in the filly were lethargy, intermittent fever and abdominal distention, primarily due to a markedly enlarged liver. Similar signs are reported in children and adults with hepatoblastoma.1,2 The tumor is highly vascularized and some human patients can present with hemoperitoneum or gastrointestinal hemorrhage. Hemorrhagic intestinal contents were seen in this horse on post-mortem examination, but a cause for the hemorrhage was not identified and it could be due to congestion from the erythrocytosis. As seen in this case, ultrasonographic imaging findings are not specific and can be seen with other hepatic tumors, aneurysms or abscesses in people. However, multiphase computerized tomography or magnetic resonance imaging may provide a definitive tumor diagnosis (albeit not specific for tumor type) due to the vascularized nature of hepatic tumors, which also retain contrast.1,2
In this case, the horse had a large tumor involving the liver as well as intrahepatic metastases and evidence of thrombosis with coagulative necrosis (both tumoral invasion into vessels and thrombosis would explain the intraluminal material seen on imaging in this case). In human patients, tumors can be single or multifocal and can metastasize to other organs, including lymph nodes and lungs, locally invade abdominal organs, or disseminate throughout the peritoneum.1-3 Vascular invasion with thrombosis can also be seen in some human patients with hepatoblastoma and is associated with a poor prognosis.1,3
This case had several clinical pathologic changes that have been recognized in other cases of hepatoblastoma in horses and in people, including secondary inappropriate erythrocytosis, thrombocytosis and hypoglycemia (Question 3).15-17 In this case, the source of the erythropoietin inciting the inappropriate erythrocytosis was likely the tumor itself, since the liver is a normal source of erythropoietin production. However, immunostaining of the tumor for erythropoietin was not performed to confirm this hypothesis. Similarly, thrombopoietin is produced in the liver and tumor-associated production of thrombopoietin may be responsible for the thrombocytosis. Alternatively, the thrombocytosis could be cytokine-mediated, since the horse did have evidence of an acute phase response (low albumin, iron and percentage transferrin saturation and high fibrinogen, reflecting negative and positive acute phase responses, respectively) as well as likely antigenic stimulation (increased globulins on the chemistry profile). Hypoglycemia is a rare manifestation of hepatoblastoma in people,15 although it is commonly seen in Beckman-Weiderman syndrome, a genetic defect that predisposes human beings to hepatoblastoma.18 It is possible that the hypoglycemia in this case was due to tumor-associated secretion of insulin-like growth factor. The low MCV in this horse can be attributed to its young age and possibly the hyponatremia (artifactual shrinkage of red blood cells in the hematology analyzer). The cause for the decrease in sodium and some of the decrease in chloride is likely fluid losses (from the gastrointestinal tract with diarrhea) and isotonic fluid supplementation (biochemical testing was done after overnight treatment), combined with an antidiuretic hormone-mediated dilutional effect. The chloride is slightly low, which is disproportionate to the decrease in sodium. This, with the low bicarbonate, supports a mild hyperchloremic acidosis, which may be primary (isotonic fluid administration) or secondary to a respiratory alkalosis. The high AST activity with a normal CK activity likely reflects hepatocellular injury, however the SDH activity was surprisingly normal. We have seen this pattern of changes in other horses with liver injury and it remains difficult to explain, considering the cytosolic location of both AST and SDH. It is possible the SDH is being inactivated for some reason. The high GGT activity can be attributed to the biliary hyperplasia, which is likely a response to localized cholestasis (potential compression by the adjacent tumor). The high normal bile acid concentration, despite laboratory evidence of cholestasis (high direct bilirubin on the chemistry panel, bile casts on the impression smears, and bile plugs on the histologic sections), indicates that function of the remaining hepatocytes, at least in relation to bile acid production and excretion, is intact. The prolonged prothrombin time, with the normal activated partial thromboplastin time, could indicate a degree of hepatic dysfunction, with respect to production or activation of factor VII, or possibly disseminated intravascular coagulation precipitated by the tumor or necrosis. The cholestasis does not seem severe enough to attribute the prolonged prothrombin time to vitamin K deficiency.
The cytologic findings with the young age of the animal were supportive of a hepablastoma. A hepatic carcinoid was not considered as a differential diagnosis because the tumor cells lacked the characteristic packeting and ruptured appearance (“naked nuclei” neoplasm) of typical endocrine tumors. However, tumors do not always follow classic or expected morphologic patterns. The diagnosis of hepatoblastoma in this case was confirmed by lack of immunostaining for neuroendocrine markers and positive, albeit weak, reaction for alpha-fetoprotein, which is a marker of immature hepatocytes. Serum testing for alpha-fetoprotein is performed in human patients as part of risk stratification, with low values (<100 ng/mL) being a negative prognostic indicator.2,3
A recent histologic scheme classifies 2 basic types of hepatoblastoma: Epithelial and epithelial with mesenchymal components, including bone, cartilage, and muscle. The epithelial types are more common than the mixed epithelial-mesenchymal type. The epithelial type is further subclassified based on morphologic features into fetal (well-differentiated, low mitotic activity), “crowded fetal” (increased mitoses), pleomorphic poorly differentiated, epithelial mixed, embryonal, macrotrabecular epithelial, cholangioblastic, and small cell undifferentiated. This histologic classification is of prognostic relevance, because the fetal and small cell undifferentiated types have the best (surgically resectable) and poorest prognosis, respectively. However, mixed types are more common than pure subtypes and pure subtypes may reflect lack of sampling versus a true pure tumor3,19 The hepatoblastoma in this case had features most characteristic of an embryonal type with small cells, a high mitotic rate, and acinar-like arrangements.19,20 A mesenchymal component was not evident.
In human patients, hepatoblastoma is treated with surgical excision (depending on the tumor type and extent of vascular invasion) and chemotherapy, with transarterial embolization being used to treat hemorrhagic episodes.1,3 The prognosis is better in children than in adults, with >90% survival in children with low risk stratification and 25-40% survival in those with high risk. In contrast, overall survival in adults is an average of 5 months with 30% survival at one year.1,3
References
- Celotti A, D’Amico G, Ceresoli M, et al. Hepatoblastoma of the adult: A systematic review of the literature. Surg Oncol 2016;25:339-347.
- Hiyama E. Pediatric hepatoblastoma: diagnosis and treatment. Transl Pediatr 2014;3:293-299.
- Bell D, Ranganathan S, Tao J, et al. Novel Advances in Understanding of Molecular Pathogenesis of Hepatoblastoma: A Wnt/beta-Catenin Perspective. Gene Expr 2017;17:141-154.
- Gatta G, Ferrari A, Stiller CA, et al. Embryonal cancers in Europe. Eur J Cancer 2012;48:1425-1433.
- Beeler-Marfisi J, Arroyo L, Caswell JL, et al. Equine primary liver tumors: a case series and review of the literature. J Vet Diagn Invest 2010;22:174-183.
- de Vries C, Vanhaesebrouck E, Govaere J, et al. Congenital ascites due to hepatoblastoma with extensive peritoneal implantation metastases in a premature equine fetus. J Comp Pathol 2013;148:214-219.
- Loynachan AT, Bolin DC, Hong CB, et al. Three equine cases of mixed hepatoblastoma with teratoid features. Vet Pathol 2007;44:211-214.
- Gold JR, Warren AL, French TW, et al. What is your diagnosis? Biopsy impression smear of a hepatic mass in a yearling Thoroughbred filly. Vet Clin Pathol 2008;37:339-343.
- Ano N, Ozaki K, Nomura K, et al. Hepatoblastoma in a cat. Vet Pathol 2011;48:1020-1023.
- Watt BC, Cooley AJ, Darien BJ. Congenital hepatoblastoma in a neonatal alpaca cria. Can Vet J 2001;42:872-874.
- Eichenmuller M, Trippel F, Kreuder M, et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J Hepatol 2014;61:1312-1320.
- Jia D, Dong R, Jing Y, et al. Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 2014;60:1686-1696.
- Comerford SA, Hinnant EA, Chen Y, et al. Hepatoblastoma modeling in mice places Nrf2 within a cancer field established by mutant beta-catenin. JCI Insight 2016;1:e88549.
- Akhavanfard S, Vargas SO, Han M, et al. Inactivation of the tumor suppressor WTX in a subset of pediatric tumors. Genes Chromosomes Cancer 2014;53:67-77.
- Madabhavi I, Patel A, Choudhary M, et al. Paraneoplastic recurrent hypoglycaemic seizures: an initial presentation of hepatoblastoma in an adolescent male-a rare entity. Case Rep Pediatr 2014;2014:104543.
- Axon JE, Russell CM, Begg AP, et al. Erythrocytosis and pleural effusion associated with a hepatoblastoma in a Thoroughbred yearling. Aust Vet J 2008;86:329-333.
- Lennox TJ, Wilson JH, Hayden DW, et al. Hepatoblastoma with erythrocytosis in a young female horse. J Am Vet Med Assoc 2000;216:718-721, 685.
- Shuman C, Beckwith JB, Weksberg R. Beckwith-Wiedemann Syndrome In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews(R). Seattle (WA), 1993.
- Lopez-Terrada D, Alaggio R, de Davila MT, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 2014;27:472-491.
- Rowland JM. Hepatoblastoma: assessment of criteria for histologic classification. Med Pediatr Oncol 2002;39:478-483.
Authored by: T Stokol.